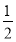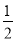��Ŀ����
�������ݶ�Ӧ���ʵ��۵㣬�ݴ����������ж��д�����ǣ� ����
Na2O | NaCl | AlF3 | AlCl3 |
920 �� | 801 �� | 1 292 �� | 190 �� |
BCl3 | Al2O3 | CO2 | SiO2 |
��107 �� | 2 073 �� | ��57 �� | 1 723 �� |
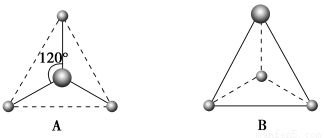
A�����Ļ�����ľ����������Ӿ���
B������ֻ��BCl3�ɱ��Ƿ��Ӿ���
C��ͬ��Ԫ�ص���������γɲ�ͬ���͵ľ���
D����ͬ��Ԫ�ص���������γ���ͬ���͵ľ���
B
���������ɱ���֪��AlCl3��BCl3��CO2�ǹ��ۻ��������γɷ��Ӿ��壻SiO2��ԭ�Ӿ��壻���������Ӿ��塣

 ��ҵ����ϵ�д�
��ҵ����ϵ�д������ס�����ͬ��Ԫ�أ�����Ԫ�ص��ʼ��仯������ũҩ�����������ȷ�������ҪӦ�á���ش��������⣺
��1����ԭ�ӵĺ�������Ų�ʽΪ_____________________________________��
��2��K3[Fe��CN��6]������Fe3����CN��֮��ļ���Ϊ________���û�ѧ���ܹ��γɵ�ԭ����______________________________________________________��
��3��NH4���е�ԭ�ӵ��ӻ�����Ϊ________��NH4���Ŀռ乹��Ϊ________��
��4����֪��
| CH4 | SiH4 | NH3 | PH3 |
�е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
�ֽ��¶ȣ�K�� | 873 | 773 | 1 073 | 713.2 |
�����ϱ����������ʵ�������ݣ���ش�
CH4��SiH4�Ƚϣ�NH3��PH3�Ƚϣ��е�ߵ͵�ԭ����___________________________________________________________________________��
CH4��SiH4�Ƚϣ�NH3��PH3�Ƚϣ��ֽ��¶ȸߵ͵�ԭ����_________________________________________________________________________��
����������ݺ����жϣ�һ��ѹǿ��HF��HCl�Ļ�����彵��ʱ________��Һ����
��5���縺�ԣ���X��ʾ��Ҳ��Ԫ�ص�һ����Ҫ���ʣ��±�����8��Ԫ�صĵ縺����ֵ��
Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
�縺�� | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 0.8 |
��ش������й����⣺
���Ƹ�Ԫ�صĵ縺�Ե�ȡֵ��Χ��________<X<________��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlCl3���γɵĻ�ѧ�������ͼ������ɣ�__________________________��