��Ŀ����
������ͼ��ʾ��װ����ȡ�϶����ı�����ˮ���ⶨ������ˮ��pH���ش��й�����
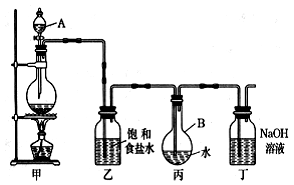
��1��д���йػ�ѧ����ʽ��װ�üף�________________________��װ�ö���_________________ ��
��2��֤����ˮ�ѱ��͵�������_________________��
��3����ȡ����ʱ��װ�ñ���Һ���к�����������___________��ˮ���ӳ��⣩��װ���ҵ�������_____________��
��4�������¸Ľ���ʩ���飬����������
��2��֤����ˮ�ѱ��͵�������_________________��
��3����ȡ����ʱ��װ�ñ���Һ���к�����������___________��ˮ���ӳ��⣩��װ���ҵ�������_____________��
��4�������¸Ľ���ʩ���飬����������
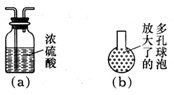
����װ���Һͱ�֮��������ͼ��a����ʾ��װ�ã�����Ϊ���ޱ�Ҫ��_______________________________��
����װ�ñ��ij������¿ڴ�������ͼ��b����ʾ�Ķ�����ݣ��������ĺô��ǣ�_________________________��
������ȥװ���ң�ֱ�ӽ�װ�üͱ���������������ʵ������Ӱ����____________��
����װ�ñ��ij������¿ڴ�������ͼ��b����ʾ�Ķ�����ݣ��������ĺô��ǣ�_________________________��
������ȥװ���ң�ֱ�ӽ�װ�üͱ���������������ʵ������Ӱ����____________��
��1��MnO2+4HCl(Ũ) MnCl2��Cl2����2H2O��2NaOH+Cl2=NaCl+NaClO+H2O
MnCl2��Cl2����2H2O��2NaOH+Cl2=NaCl+NaClO+H2O
��2�����еĵ������������ˮ�棬��Һ���Ϸ���������ɫ���塣
��3��Cl2�� ��ȥ�����е��Ȼ�������
��4�����ޣ�������������ˮ�ĽӴ���������������ܽ⣻��ʹ�ⶨ��pH��С
 MnCl2��Cl2����2H2O��2NaOH+Cl2=NaCl+NaClO+H2O
MnCl2��Cl2����2H2O��2NaOH+Cl2=NaCl+NaClO+H2O ��2�����еĵ������������ˮ�棬��Һ���Ϸ���������ɫ���塣
��3��Cl2�� ��ȥ�����е��Ȼ�������
��4�����ޣ�������������ˮ�ĽӴ���������������ܽ⣻��ʹ�ⶨ��pH��С

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ
 ijͬѧ��ʵ��������ͼ��ʾ��ʵ��װ����ȡ���ռ�����İ�������ش��������⣮
ijͬѧ��ʵ��������ͼ��ʾ��ʵ��װ����ȡ���ռ�����İ�������ش��������⣮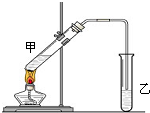 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
