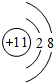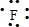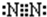��Ŀ����
A��D��ԭ��������20���ڵ�Ԫ�أ������ʻ�ṹ��Ϣ���±�| Ԫ�� | A | B | C | D |
| ���� �� �ṹ ��Ϣ | �䵥�ʺͻ��������ɫ��Ϊ��ɫ | ���⻯���ˮ��Һ�ܸ�ʴ���� | ʪ��ĺ�ɫʯ����ֽ������ͼ��⻯����� | ��ԭ�Ӻ�����13���˶�״̬�ĵ��� |
��1��A���ӵĵ����Ų�ʽΪ______��Bԭ�Ӻ�����ӵĹ����ʾʽΪ______��
��2����B���⻯��������������ͬ��4��������______��
��3��C�ĵ����ر��ȶ���ԭ������______��C����̬�⻯���ˮ��Һ�д��ڵ�ƽ�⣺______��
��4����ҵ����ȡ D�ĵ��ʵĻ�ѧ����ʽΪ______ 4Al+3O2��
���𰸡������������䵥�ʺͻ��������ɫ��Ϊ��ɫ����AΪNa��
�������⻯���ˮ��Һ�ܸ�ʴ��������BΪF��
����ʪ��ĺ�ɫʯ����ֽ������ͼ��⻯���������CΪN��
������ԭ�Ӻ�����13���˶�״̬�ĵ��ӣ���CΪAl��
Ȼ���������ش�
����⣺��1��Na+�ĵ����Ų�ʽΪ��1s22s22p6�� ������ӵĹ����ʾʽΪ��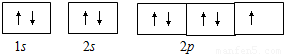
�ʴ�Ϊ��1s22s22p6��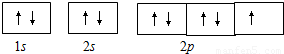 ��
��
��2��HF������������Ϊ10��������10���������У�Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+
�ʴ�Ϊ��Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+�����е�����4����ɣ�
��3�����������е�����Ϊ���������������̣����ܴ��ι����Զ��ѣ����Ե����ر��ȶ���NH3��ˮ��Һ�д��ڵ�ƽ���У�NH3+H2O?NH3?H2O?NH4++OH-��
�ʴ��У����������е�����Ϊ���������������̣����ܴ��ι����Զ��ѣ�NH3+H2O?NH3?H2O?NH4++OH-��
��4����ҵ����ȡ�������õ�ⷨ��2Al2O3 �����ڣ� 4Al+3O2�����ʴ�Ϊ��2Al2O3 �����ڣ�
4Al+3O2�����ʴ�Ϊ��2Al2O3 �����ڣ� 4Al+3O2����
4Al+3O2����
���������⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�ѶȲ�����ȷ�ƶ�Ԫ�ص������ǽ����Ĺؼ���Ȼ�����Ԫ�ػ�����֪ʶ���н��
�������⻯���ˮ��Һ�ܸ�ʴ��������BΪF��
����ʪ��ĺ�ɫʯ����ֽ������ͼ��⻯���������CΪN��
������ԭ�Ӻ�����13���˶�״̬�ĵ��ӣ���CΪAl��
Ȼ���������ش�
����⣺��1��Na+�ĵ����Ų�ʽΪ��1s22s22p6�� ������ӵĹ����ʾʽΪ��

�ʴ�Ϊ��1s22s22p6��
 ��
�� ��2��HF������������Ϊ10��������10���������У�Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+
�ʴ�Ϊ��Ne��H2O��NH3��CH4��OH-��F-��O2-��N3-��Na+��Mg2+��Al3+��H3O+��NH4+�����е�����4����ɣ�
��3�����������е�����Ϊ���������������̣����ܴ��ι����Զ��ѣ����Ե����ر��ȶ���NH3��ˮ��Һ�д��ڵ�ƽ���У�NH3+H2O?NH3?H2O?NH4++OH-��
�ʴ��У����������е�����Ϊ���������������̣����ܴ��ι����Զ��ѣ�NH3+H2O?NH3?H2O?NH4++OH-��
��4����ҵ����ȡ�������õ�ⷨ��2Al2O3 �����ڣ�
 4Al+3O2�����ʴ�Ϊ��2Al2O3 �����ڣ�
4Al+3O2�����ʴ�Ϊ��2Al2O3 �����ڣ� 4Al+3O2����
4Al+3O2�������������⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�ѶȲ�����ȷ�ƶ�Ԫ�ص������ǽ����Ĺؼ���Ȼ�����Ԫ�ػ�����֪ʶ���н��

��ϰ��ϵ�д�
 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
A��D��ԭ��������20���ڵ�Ԫ�أ������ʻ�ṹ��Ϣ���±�
����ݱ��е���Ϣ�ش��������⣺
��1��A���ӵĽṹʾ��ͼ ��Bԭ�ӵĵ���ʽ ��C�ĵ��ʵĵ���ʽΪ ��
��2����B���⻯��������������ͬ��4������ �� �� �� ��
��3��C����̬�⻯���ˮ����ĵ��뷽��ʽ�� ��
��4����Ӧ���Ӱ뾶��С�Ƚϣ�A D��
| Ԫ�� | A | B | C | D |
| ���ʻ� �ṹ��Ϣ | �䵥�ʺͻ��������ɫ��Ϊ��ɫ | ���⻯���ˮ��Һ�ܸ�ʴ���� | �䵥���ڿ��������������� | �ؿ��к������Ľ���Ԫ�� |
��1��A���ӵĽṹʾ��ͼ ��Bԭ�ӵĵ���ʽ ��C�ĵ��ʵĵ���ʽΪ ��
��2����B���⻯��������������ͬ��4������ �� �� �� ��
��3��C����̬�⻯���ˮ����ĵ��뷽��ʽ�� ��
��4����Ӧ���Ӱ뾶��С�Ƚϣ�A D��