��Ŀ����
��10�֣���1����֪25��ʱ��1 g H2����������ȫȼ������Һ̬ˮ���ų�����142.9kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��__________________��
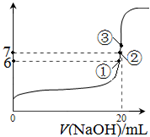
��2�����ø÷�Ӧ���һ��ȼ�ϵ�أ�������������Һ���������Һ���ö��ʯī���缫���ڵ缫�Ϸֱ���������������д�������ĵ缫��Ӧʽ��_________________________��
�õ�ع���һ��ʱ���������������Һ��pHֵ��______������С�䣩��
��3������ͬ�����£�1 mol H2ֱ��ȼ�ղ�������������Ƴ�ȼ�ϵ�ز���������______���ֱ��ȼ�նࡢȼ�ϵ�ضࡢһ���ࣩ��
��4��������һ��ʮ������������Դ��Ŀǰ�������Ŵ�������е�̫������Һ�������⣬����������е�ܵ͵�ԭ��_____________________��
��1��2H2(g)��O2(g)===2H2O(1) ��H����571.6kJ��mol��1�����������Ĵ�Ҳ���֣�
��2��H2��2OH����2e��===2H2O ��� ��3��һ����
��4����������Է�������С�����Ӽ�������С���ʷе�͡�
����������1�������Ȼ�ѧ����ʽ����д��1 g H2����������ȫȼ������Һ̬ˮ���ų�����142.9kJ����1mol����ȼ�շų���������142.9kJ��2��285.8Kj�������Ȼ�ѧ����ʽΪ2H2(g)��O2(g)===2H2O(1) ��H����571.6kJ��mol��1�����������Ĵ�Ҳ���֣�
��2����ԭ����и�����ʧȥ���ӣ�����������Ӧ�ġ���������ͨ�븺�����缫��ӦʽΪH2��2OH����2e��===2H2O������ͨ���������缫��ӦʽΪO2��2H2O +4e��===4OH����������������pH����
��3�����������غ��֪��1 mol H2ֱ��ȼ�ղ�������������Ƴ�ȼ�ϵ�ز�������������ͬ�ġ�
��4�������γɵľ����Ƿ��Ӿ��壬��Է���������С����˷��Ӽ���������С�����Էе�ܵ͡�

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�