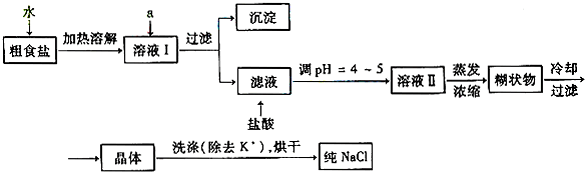
��Ŀ����
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϣ�
��1����ʳ�γ���������Ca2+��Mg2+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba��NO3��2��Һ
������ȥ��Һ���е�Ca2+��Mg2+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ______��ֻ�ѧʽ����
�ڹ���֮ǰ����������SO42-�ѳ�ȥ��______��
�۹�������Ҫ�IJ���������______��
�������������pH�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����______��
��2�����ᴿ��NaCl����250mL 2.00mol-L-1NaCl��Һ��
������������ҩ����������漰�����⣬����______�����������ƣ���
�ڼ������Ƴ�NaOH����Ϊ______g��
�����в�������ȷ˳���ǣ�����ĸ��ʾ��______��
A��ҡ�� B������ C��ϴ�� D������ E���ܽ� F����Һ G��װƿ
�����в�����������ҺŨ���к�Ӱ�죬�ں�������д��ƫ�ߡ���ƫ�͡�����Ӱ�족��
A ����ʱ��������ƿ�̶��ߣ�______��B ����ʱ�������⣺______��
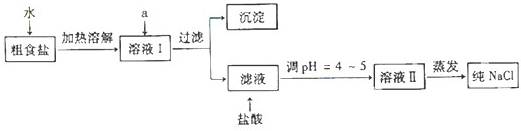
��1����ʳ�γ���������Ca2+��Mg2+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba��NO3��2��Һ
������ȥ��Һ���е�Ca2+��Mg2+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ______��ֻ�ѧʽ����
�ڹ���֮ǰ����������SO42-�ѳ�ȥ��______��
�۹�������Ҫ�IJ���������______��
�������������pH�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����______��
��2�����ᴿ��NaCl����250mL 2.00mol-L-1NaCl��Һ��
������������ҩ����������漰�����⣬����______�����������ƣ���
�ڼ������Ƴ�NaOH����Ϊ______g��
�����в�������ȷ˳���ǣ�����ĸ��ʾ��______��
A��ҡ�� B������ C��ϴ�� D������ E���ܽ� F����Һ G��װƿ
�����в�����������ҺŨ���к�Ӱ�죬�ں�������д��ƫ�ߡ���ƫ�͡�����Ӱ�족��
A ����ʱ��������ƿ�̶��ߣ�______��B ����ʱ�������⣺______��

��1����Ҫ��ȥSO42-��ֻ��ѡBaCl2��Һ����ѡ��Ba��NO3��2���������µ�����NO3-����ѡ��NaOH��Һ��ȥMg2+��Fe3+��Һ�����ѡ��Na2CO3��Һ��ȥCa2+���˴�����ѡ��K2CO3��Һ������������µ�K+������HCl��ȥ������CO32-��Na2CO3��Һ���ܼ���BaCl2��Һǰ�����������Ba2+���ʴ�Ϊ��BaCl2��NaOH��Na2CO3��
��ȡ��������Һ���Թ��У������еμ��Ȼ�����Һ�����Ƿ��г������ɣ���û�г�����������������ѳ�����ȫ���ʴ�Ϊ��ȡ��������Һ���Թ��У������еμ��Ȼ�����Һ�����Ƿ��г������ɣ���û�г�����������������ѳ�����ȫ��
�۹���װ����Ҫ�õ��IJ���������©�����ձ������������ʴ�Ϊ��©�����ձ�����������
�����������pH�ٹ��ˣ����ɵ�Mg��OH��2��CaCO3��BaCO3�������ᷴӦ�����˺����ᴿ�Ȼ��ƣ�
�ʴ�Ϊ�����ɵ�Mg��OH��2��CaCO3��BaCO3�������ᷴӦ���Ӷ�Ӱ���Ƶ�ʳ�εĴ��ȣ�
��2�������ᴿ��NaCl����250mL 2.00mol?L-1NaCl��Һ����Ҫ���������ձ�����������250ml����ƿ����ͷ�ιܡ�������ƽ�������ձ���������������ʵ���õ�������Ҫ250ml����ƿ����ͷ�ιܡ�������ƽ���ʴ�Ϊ��250ml����ƿ����ͷ�ιܡ�������ƽ��
������250mL 2.00mol?L-1NaCl��Һ���������ʵ���=0.25L��2.00mol/L=0.5mol���Ȼ�������=0.5mol��58.5g/mol=29.25g����������ƽֻ�ܳ�����0.1g��������Ҫ�����Ȼ�������29.3g��
�ʴ�Ϊ��29.3��
����Һ���Ʋ����Ǽ��㡢�������ܽ⡢ת�ơ�ϴ��ת�ƣ���ˮ���ݡ�ҡ�ȡ�װƿ�����Բ�������ȷ˳����B��E��F��C��D��A��G���ʴ�Ϊ��B��E��F��C��D��A��G��
��A������ʱ��������ƿ�̶��ߣ����������ǿ̶ȶ�Һ�棬���Ӽ�ˮ�����̶��ߣ�Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
B������ʱ�������⣬���������������Ȼ������������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��ȡ��������Һ���Թ��У������еμ��Ȼ�����Һ�����Ƿ��г������ɣ���û�г�����������������ѳ�����ȫ���ʴ�Ϊ��ȡ��������Һ���Թ��У������еμ��Ȼ�����Һ�����Ƿ��г������ɣ���û�г�����������������ѳ�����ȫ��
�۹���װ����Ҫ�õ��IJ���������©�����ձ������������ʴ�Ϊ��©�����ձ�����������
�����������pH�ٹ��ˣ����ɵ�Mg��OH��2��CaCO3��BaCO3�������ᷴӦ�����˺����ᴿ�Ȼ��ƣ�
�ʴ�Ϊ�����ɵ�Mg��OH��2��CaCO3��BaCO3�������ᷴӦ���Ӷ�Ӱ���Ƶ�ʳ�εĴ��ȣ�
��2�������ᴿ��NaCl����250mL 2.00mol?L-1NaCl��Һ����Ҫ���������ձ�����������250ml����ƿ����ͷ�ιܡ�������ƽ�������ձ���������������ʵ���õ�������Ҫ250ml����ƿ����ͷ�ιܡ�������ƽ���ʴ�Ϊ��250ml����ƿ����ͷ�ιܡ�������ƽ��
������250mL 2.00mol?L-1NaCl��Һ���������ʵ���=0.25L��2.00mol/L=0.5mol���Ȼ�������=0.5mol��58.5g/mol=29.25g����������ƽֻ�ܳ�����0.1g��������Ҫ�����Ȼ�������29.3g��
�ʴ�Ϊ��29.3��
����Һ���Ʋ����Ǽ��㡢�������ܽ⡢ת�ơ�ϴ��ת�ƣ���ˮ���ݡ�ҡ�ȡ�װƿ�����Բ�������ȷ˳����B��E��F��C��D��A��G���ʴ�Ϊ��B��E��F��C��D��A��G��
��A������ʱ��������ƿ�̶��ߣ����������ǿ̶ȶ�Һ�棬���Ӽ�ˮ�����̶��ߣ�Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
B������ʱ�������⣬���������������Ȼ������������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�

��ϰ��ϵ�д�
�����Ŀ