��Ŀ����
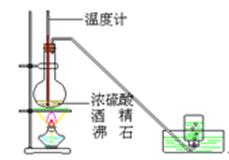
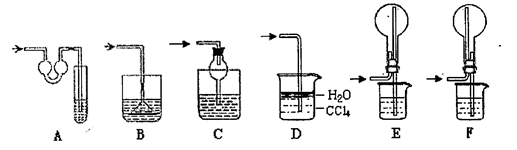
��23�֣�����װ��ͼ�У����������ķ���װ�ã��¡����Ǿ��������װ�ã�����װ��˿������Ӧ��ŵĵײ����ػ�ɫ����ۼ����������ն��������װ�á�
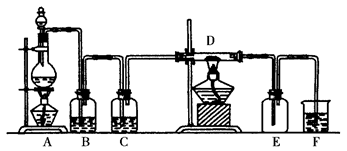
��1��ʵ��ǰ�������װ�������Եķ����ǣ�
��2����װ���з�Һ©����װʲôҩƷ ����ƿ��װʲôҩƷ ��
��3��B����ʢװ���� �������� ��
������ʢװ���� �������� ��
��4��д�������ġ�������װ���з�����Ӧ�Ļ�ѧ����ʽ
��5����ѧʵ���м����Ƿ���Cl2��������ʪ��ĵ���-KI��ֽ������Cl2�������ɹ۲쵽�������� ��д����Ӧ����ʽ ��

��1��ʵ��ǰ�������װ�������Եķ����ǣ�

��2����װ���з�Һ©����װʲôҩƷ ����ƿ��װʲôҩƷ ��
��3��B����ʢװ���� �������� ��
������ʢװ���� �������� ��
��4��д�������ġ�������װ���з�����Ӧ�Ļ�ѧ����ʽ
��5����ѧʵ���м����Ƿ���Cl2��������ʪ��ĵ���-KI��ֽ������Cl2�������ɹ۲쵽�������� ��д����Ӧ����ʽ ��
��1���رշ�Һ©���������þƾ�����Բ����ƿ�����ձ��еĵ��ܿ�������ð��ʱ��ֹͣ���ȣ������ܿ��γ�һС��ˮ������֤�����������ã�3�֣�
��2��Ũ���� MnO2 ��ÿ��1�֣���2�֣�
��3������ʳ��ˮ ��ȥ������������HCl
Ũ���� ��ȥ������������ˮ���� ��ÿ��2�֣���8�֣�
��4��MnO2+4HCl(Ũ)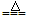 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
2Fe + 3Cl2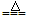 2FeCl3
2FeCl3
2NaOH+Cl2===NaCl+NaClO+H2O ��ÿ��2�֣���6�֣�
��5����ֽ����ɫ 2KI+Cl2===2KCl+I2 ��ÿ��2�֣���4�֣�
��2��Ũ���� MnO2 ��ÿ��1�֣���2�֣�
��3������ʳ��ˮ ��ȥ������������HCl
Ũ���� ��ȥ������������ˮ���� ��ÿ��2�֣���8�֣�
��4��MnO2+4HCl(Ũ)
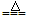 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O2Fe + 3Cl2
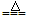 2FeCl3
2FeCl32NaOH+Cl2===NaCl+NaClO+H2O ��ÿ��2�֣���6�֣�
��5����ֽ����ɫ 2KI+Cl2===2KCl+I2 ��ÿ��2�֣���4�֣�
��

��ϰ��ϵ�д�
�����Ŀ
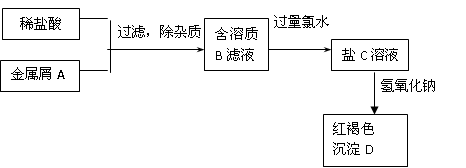

 MnCl2+2H2O+ Cl2��
MnCl2+2H2O+ Cl2��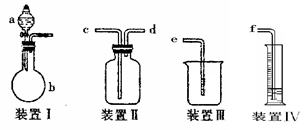
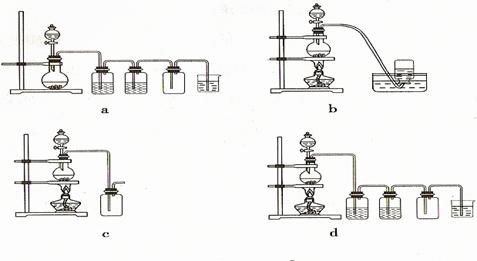
 �����
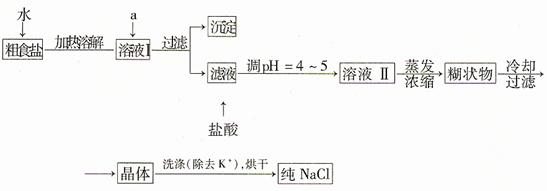
�����
 ʵ��Ŀ�ĵ��ǣ� ��
ʵ��Ŀ�ĵ��ǣ� ��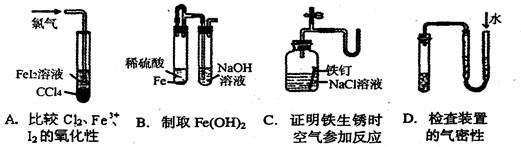
 ��3I2+3H2O+3K2SO4
��3I2+3H2O+3K2SO4