��Ŀ����
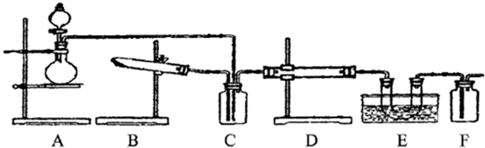
��ͼʵ��װ��ʾ��ͼ��A�Ǽ���H2��������B�Ǵ�С���˵ĵ�Բ����ƿ��C��װ�и������U�ιܡ�A����ת������D��װ�л�ԭ���۵ķ�Ӧ�ܣ�E��װ�з�̪���Թܡ�ʵ��ǰ�ȼ��װ�õ������ԡ�ʵ�鿪ʼʱ���ȹرջ���a����ȡ����ƿB����A�Ƽ���һ����Ũ���ʵ������ᣬ����H2��������Ҫ�ġ����������ڵ��ܿڴ���ȼH2��Ȼ����ͼ��ʾ������ƿB��������ƿ��H2����ƿ�м���ȼ�գ��þƾ��Ƽ��ȷ�Ӧ��D�еĻ�ԭ���ۣ���B��H2�Ļ���Ϩ�����a������ͨ����Ӧ��D�����ձ�E�У�ʹ��̪��Һ�ʺ�ɫ��
��ش��������⡣
��1��ʵ��ǰ��μ������װ�õ������ԣ� ��
��2������б�Ҫ��������ָ����
��3���������ȼ���D���a��Ŀ����
��4��д��B��D�зֱ�����Ӧ�Ļ�ѧ����ʽ��B�� ��
D�� ��
��5��C�еĸ������������ ���ø������������ ��
��1�����ֶμ��飩�ڳ���©���з�������ˮ��ʹˮ��պý�û����©���¶ˣ��ر�a��������ˮ��©����Һ������Թ���Һ�棬��һ��ʱ���ڱ��ֲ��䣻��E�м�ˮ��û�����ܿڣ����Թ�D�ĵײ����ȣ���E���ܿ��������ݳ���ֹͣ���ȣ�����һ���ȶ���ˮ��������װ�ò�©����
��2�����H2�Ĵ��ȣ�����ˮ���ռ�һ�Թ�H2����Ĵָ��ס���ƽ��ƾ��ƻ��棬���û�м���ı���������ʾ�ϴ�����
��3�����ȴﵽ�����Ļ��ԣ���ʹ����ͨ�뼴��Ӧ�����ԭ�������ʡ�
��4��2H2 + O2 ![]() 2H2O��N2+3H2=2NH3
2H2O��N2+3H2=2NH3
(5)��ʯ�ң�����������ˮ������HCl������