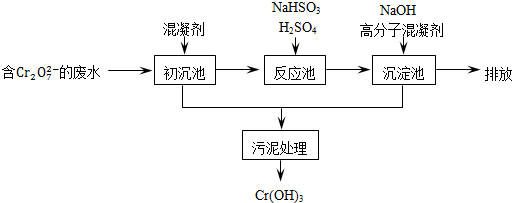
��Ŀ����
�����ͷ�ˮ���ؽ���Ԫ�ظ��Ķ��ԣ��ɽ�Cr2O72-ת��ΪCr��OH��3������ȥ����֪��
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe2+ | 7.0 | 9.0 |
| Fe3+ | 1.9 | 3.2 |
| Cr3+ | 6.0 | 8.0 |
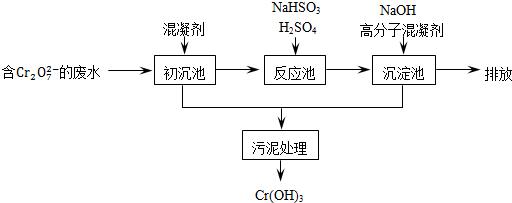
�ٳ������м���Ļ�������K2SO4�qAl2��SO4��3�q24H2O�������ӷ���ʽ��ʾ�䷴Ӧԭ����______��
�ڷ�Ӧ���з�����Ҫ��Ӧ�����ӷ���ʽ��Cr2O72-+3HSO3-+5H+�T2Cr3++3SO42-+4H2O�����ݡ����������͡��кͷ�����ԭ������������м���NaOH��Һ���˹����з�����Ҫ��Ӧ�����ӷ���ʽ��______��֤��Cr3+������ȫ�ķ�����______��

��2����ҵ���õ�ⷨ�������� Cr2O72-��ˮ��ʵ����������ͼģ�����Cr2O72-�ķ�ˮ��������Ӧʽ��Fe-2e-�TFe2+��������Ӧʽ��2H++2e-�TH2����Fe2+��������Һ�е�Cr2O72-��Ӧ�����ӷ���ʽ��______���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ����______���õ�ⷨ��������Һ��0.01mol Cr2O72-ʱ�����ٵõ�������������______ g��
�⣺��1����K2SO4�qAl2��SO4��3�q24H2OΪǿ����ʣ�����Һ����ȫ���룬����Al3+��SO42-��K+��Al3+��ˮ�����������������壺Al3++3H2O=Al��OH��3�����壩+3H+��Al3++3H2O?Al��OH��3+3H+����������������������ԣ�������ˮ�е����������������ˮ����
�ʴ�Ϊ��Al3++3H2O=Al��OH��3�����壩+3H+��Al3++3H2O?Al��OH��3+3H+��
�ڸ��ݡ����������͡��кͷ�����ԭ������������м���NaOH��Һ��NaOH���H+������ӦH++OH-�TH2O��Cr3+��NaOH������ӦCr3++3OH-�TCr��OH��3����Cr��OH��3������ȫʱ��pHΪ8�����ԣ��ⶨ��Һ��pH����pH��8����֤��Cr3+������ȫ��
�ʴ�Ϊ��Cr3++3OH-�TCr��OH��3����H++OH-�TH2O���ⶨ��Һ��pH����pH��8����֤��Cr3+������ȫ��
��2������������Cr2O72-����������ԭ��Ӧ������ԭΪCr3+Ȼ������Cr��OH��3�������ظ��������ǿ�����ԣ��ܽ����ɵ�������������Ϊ���ۣ���6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O�����ŵ����У���Һ��c��H+�� ���٣�������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ������Fe��OH��3��Cr��OH��3�������������������������ɳ�����ȫ������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3��֪0.01mol Cr2O72-��������0.02molCr��OH��3��0.06molFe��OH��3�����ٵõ�������������0.02mol��103g/mol+0.06mol��107g/mol=8.48g��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��������Ӧ������ˮ�е�H+��������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ��8.48��
��������1�������������������������������������
�ڷ�Ӧ���з�����Ҫ��Ӧ�����ӷ���ʽ��Cr2O72-+3HSO3-+5H+�T2Cr3++3SO42-+4H2O����Ӧ����Һ����H+��Cr3+����NaOH��Һ��NaOH���H+��Cr3+������Ӧ��
��2��Fe2+������Cr2O72-���ӷ���������ԭ��Ӧ����Fe3+���Ӻ�Cr3+���ӣ����ŵ����У���Һ��c��H+�� ���٣�c��OH-��Ũ��������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3�����м��㣻
���������⿼���Ϊ�ۺϣ��漰��⡢������ԭ��Ӧ����ѧ����ʽ�ļ�������⣬Ҫ����нϺõķ����ͽ���������������Ŀ�ѶȽϴ�
�ʴ�Ϊ��Al3++3H2O=Al��OH��3�����壩+3H+��Al3++3H2O?Al��OH��3+3H+��
�ڸ��ݡ����������͡��кͷ�����ԭ������������м���NaOH��Һ��NaOH���H+������ӦH++OH-�TH2O��Cr3+��NaOH������ӦCr3++3OH-�TCr��OH��3����Cr��OH��3������ȫʱ��pHΪ8�����ԣ��ⶨ��Һ��pH����pH��8����֤��Cr3+������ȫ��
�ʴ�Ϊ��Cr3++3OH-�TCr��OH��3����H++OH-�TH2O���ⶨ��Һ��pH����pH��8����֤��Cr3+������ȫ��
��2������������Cr2O72-����������ԭ��Ӧ������ԭΪCr3+Ȼ������Cr��OH��3�������ظ��������ǿ�����ԣ��ܽ����ɵ�������������Ϊ���ۣ���6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O�����ŵ����У���Һ��c��H+�� ���٣�������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ������Fe��OH��3��Cr��OH��3�������������������������ɳ�����ȫ������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3��֪0.01mol Cr2O72-��������0.02molCr��OH��3��0.06molFe��OH��3�����ٵõ�������������0.02mol��103g/mol+0.06mol��107g/mol=8.48g��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��������Ӧ������ˮ�е�H+��������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ��8.48��
��������1�������������������������������������
�ڷ�Ӧ���з�����Ҫ��Ӧ�����ӷ���ʽ��Cr2O72-+3HSO3-+5H+�T2Cr3++3SO42-+4H2O����Ӧ����Һ����H+��Cr3+����NaOH��Һ��NaOH���H+��Cr3+������Ӧ��
��2��Fe2+������Cr2O72-���ӷ���������ԭ��Ӧ����Fe3+���Ӻ�Cr3+���ӣ����ŵ����У���Һ��c��H+�� ���٣�c��OH-��Ũ��������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3�����м��㣻
���������⿼���Ϊ�ۺϣ��漰��⡢������ԭ��Ӧ����ѧ����ʽ�ļ�������⣬Ҫ����нϺõķ����ͽ���������������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
�����Ŀ
�����ͷ�ˮ���ؽ���Ԫ�ظ��Ķ��ԣ��ɽ�Cr2O2-7ת��ΪCr��OH��3������ȥ��
��֪��
��1��ij������ˮ��������Ҫ������ͼ��ʾ��
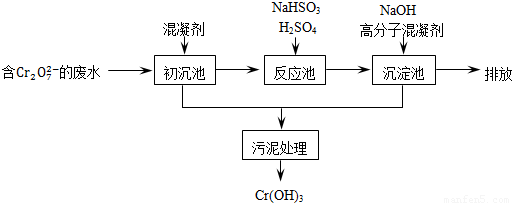
�ٳ����м���Ļ�������K2SO4?Al2��SO4��3?24H2O�������ӷ���ʽ��ʾ�䷴Ӧԭ��______��
�ڷ�Ӧ���з�����Ҫ��Ӧ�����ӷ���ʽ��Cr2O2-7+3HSO-3+5H+�T2Cr3++3SO2-4+H2O�����ݡ����������͡��кͷ�����ԭ������������м���NaOH��Һ���˹����з�����Ҫ��Ӧ�����ӷ���ʽ��______��______��֤��Cr3+������ȫ�ķ�����______��
��2����ҵ����õ�ⷨ��������Cr2O2-7��ˮ��ʵ����������ͼģ�����Cr2O2-7�ķ�ˮ��������Ӧʽ��Fe-2e-�TFe2+��������Ӧʽ��2H++2e-=H2����Fe2+��������Һ�е�Cr2O2-7��Ӧ����Cr3+��Fe3+���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ���ǣ�______��
�õ�ⷨ��������Һ��0.01molCr2O2-7ʱ�����ٵõ�������������______g��

��֪��
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe2+ | 7.0 | 9.0 |
| Fe3+ | 1.9 | 3.2 |
| Cr3+ | 6.0 | 8.0 |

�ٳ����м���Ļ�������K2SO4?Al2��SO4��3?24H2O�������ӷ���ʽ��ʾ�䷴Ӧԭ��______��
�ڷ�Ӧ���з�����Ҫ��Ӧ�����ӷ���ʽ��Cr2O2-7+3HSO-3+5H+�T2Cr3++3SO2-4+H2O�����ݡ����������͡��кͷ�����ԭ������������м���NaOH��Һ���˹����з�����Ҫ��Ӧ�����ӷ���ʽ��______��______��֤��Cr3+������ȫ�ķ�����______��
��2����ҵ����õ�ⷨ��������Cr2O2-7��ˮ��ʵ����������ͼģ�����Cr2O2-7�ķ�ˮ��������Ӧʽ��Fe-2e-�TFe2+��������Ӧʽ��2H++2e-=H2����Fe2+��������Һ�е�Cr2O2-7��Ӧ����Cr3+��Fe3+���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ���ǣ�______��
�õ�ⷨ��������Һ��0.01molCr2O2-7ʱ�����ٵõ�������������______g��