��Ŀ����
���ᡢ�������������ѧ�γ����ġ������ᡱ����͡������ᡱ�����ͭ��Ӧ��������ش��������⣺
��1������֪����ϡ������ͭ����Ӧ������ϡ�����м���H2O2�����ʹͭ�ܽ⣮�÷�Ӧ�Ļ�ѧ����ʽΪ
_____________________________________________________________________��
��2��ijͬѧδ����������Ӧ����������һ��ʵ��װ�ã�Ҳ��ʹͭ�ܿ�����ϡ���ᣮ��������ķ����л�����װ�ã�
��3����һ�������18 mol/L��Ũ�����м��������ͭƬ������ʹ֮��Ӧ������ԭ��������0.9 mol����������ʵ�����___________(���������������)100 mL����ͬѧ�������ʹʣ���ͭƬ�����ܽ⣬�������м��������Σ�������___________(����С������С�)��
��4������������ͭƬ�ֱ���������������Ũ�����ϡ���ᷴӦ�����õ�����Һǰ�߳���ɫ�����߳���ɫ��һͬѧ�����Һ�ʡ���ɫ����ԭ����������Һ��Cu2��Ũ�Ȳ�ͬ��ɣ�����Ϊ��ͬѧ���ж� _____ ���� ���ԡ� �� �����ԡ���
(1)Cu+H2O2+2HCl = CuCl 2+ 2H2O ��2�֣�
��2����ͼ����ͭΪ������������Ϊ�������Һ�ĵ���װ�á���2�֣�
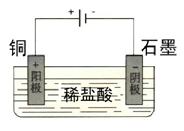
��3�����ڣ�2�֣������У�2�֣�
��4�� ���ԣ�2�֣���
����:
��

 ���ᡢ�������������ѧ�γ����ġ������ᡱ���־������������ͭ��Ӧ��������ش��������⣺
���ᡢ�������������ѧ�γ����ġ������ᡱ���־������������ͭ��Ӧ��������ش��������⣺

