��Ŀ����
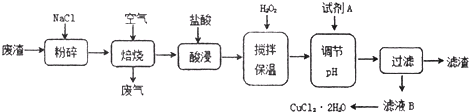
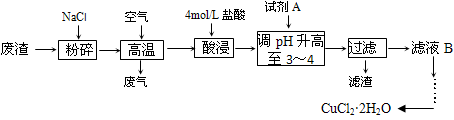
ij���������к��д���CuS���������������Ļ������ҵ���Ը÷���Ϊԭ������CuCl2��2H2O����������
��ش��������⣺
��1�������е����ڳ�ʪ�Ŀ����з���������ʴ����������ӦʽΪ___________________��
��2����������ʱCuS�����ķ�ӦΪ��___CuS + __NaCl + __O2�� ___CuCl2 + ____Na2SO4����ƽ�÷�Ӧ����ʽ��
��3����pH����Ԫ���������������ӷ���ʽΪ______________ ��
��4���Լ�A���ѡ���������������е�________������ţ���
a.NaClO b.Cl2 c.Ũ����
������__________________��
��5��������ͼ�ܽ�����ش�
��1�������е����ڳ�ʪ�Ŀ����з���������ʴ����������ӦʽΪ___________________��
��2����������ʱCuS�����ķ�ӦΪ��___CuS + __NaCl + __O2�� ___CuCl2 + ____Na2SO4����ƽ�÷�Ӧ����ʽ��
��3����pH����Ԫ���������������ӷ���ʽΪ______________ ��
��4���Լ�A���ѡ���������������е�________������ţ���
a.NaClO b.Cl2 c.Ũ����
������__________________��
��5��������ͼ�ܽ�����ش�
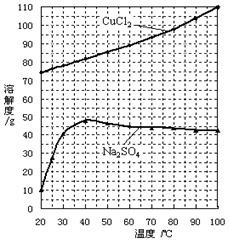
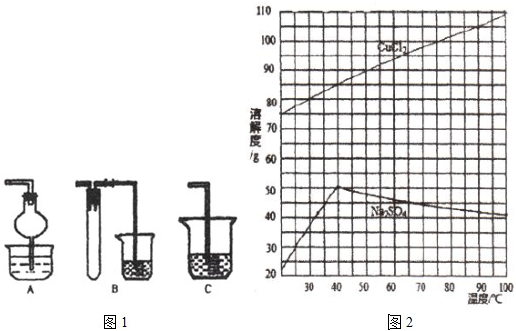
��Ϊ�˻��CuCl2��2H2O���壬����ҺB���еIJ����ǣ�����Ũ����_________����Һ��ȴ�ᾧ�����˵õ���Ʒ��
�ڡ���ȴ�ᾧ�������У�����CuCl2��2H2O����ĺ����¶Ȼ��¶ȷ�Χ��____________��
�ڡ���ȴ�ᾧ�������У�����CuCl2��2H2O����ĺ����¶Ȼ��¶ȷ�Χ��____________��
��1��2H2O+O2+4e��=4OH��
��2��1 2 2 1 1
��3��Fe3++3OH-= Fe(OH)3��
��4��a��NaClO�ܽ�Fe2������ΪFe3������NaClO��Һ�Լ��ԣ���������ҺpH
��5���ٳ��ȹ��ˣ���35��40��
��2��1 2 2 1 1
��3��Fe3++3OH-= Fe(OH)3��
��4��a��NaClO�ܽ�Fe2������ΪFe3������NaClO��Һ�Լ��ԣ���������ҺpH
��5���ٳ��ȹ��ˣ���35��40��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ