��Ŀ����
�Իش��������⣺
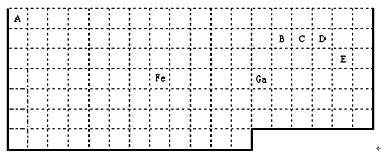
��1����д��Ԫ��o�Ļ�̬ԭ�ӵ����Ų�ʽ
��2��d���⻯������ԭ�ӵ��ӻ���ʽ��
��3��c��e��k����Ԫ�صĵ縺����ֵ��С�����˳��Ϊ
��4��f��l��q���⻯���зе���ߵ���
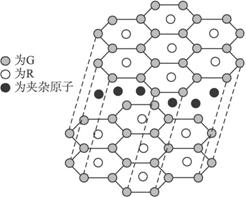
��5��jԭ���γɵľ�����jԭ�Ӹ�cԭ����1��1������϶��γɵ�jc����������ͬ����j������۵�
��2��dΪNԪ�أ����⻯��ΪNH3������Nԭ�Ӽ۲���Ӷ�����µ��Ӷ����ж����ӻ���ʽ��
��3��cΪC��dΪN��eΪO�����ߴ���ͬ���ڣ�ͬ����������ҵ縺������
��4��fΪF��lΪCl��qΪBr�����ǵ��⻯�ﶼ���ڷ��Ӿ��壬����������Է�������ȷ���е�ߵͣ�
��5��jΪSi��cΪC������Si��SiC������ԭ�Ӿ��壬���ݾ����й��ۼ������жϻ�ѧ��ǿ���������ж��۵�ߵͣ�
��2��dΪNԪ�أ����⻯��ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+
| 5-1��3 |
| 2 |
��3��cΪC��dΪN��eΪO�����ߴ���ͬ���ڣ�ͬ����������ҵ縺�����ʵ縺��C��S��O��
�ʴ�Ϊ��C��S��O��
��4��fΪF��lΪCl��qΪBr�����ǵ��⻯�ﶼ���ڷ��Ӿ��壬HBr��HCl����Է����������Ӽ���������ǿ��HBr�ķе�ϸߣ�HF����֮��������������ȷ��Ӽ�������ǿ����HF�е���ߣ��ʴ�Ϊ��HF��
��5��jΪSi��cΪC��SiC�����뾧��Si����ԭ�Ӿ��壬SiC��C-Si���ļ����Ⱦ���Si��Si-Si�����̣���������ܴ�����۷е�ߣ�
�ʴ�Ϊ�����ڣ���SiC�����뾧��Si����ԭ�Ӿ��壬SiC��C-Si���ļ����Ⱦ���Si��Si-Si�����̣���������ܴ�����۷е�ߣ�

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�(A)�����ʽṹ�����ʡ�
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ�ֻ�ѧԪ�ء�
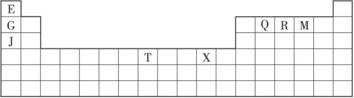
(1)T3+�ĺ�������Ų�ʽ��____________��
(2)Q��R��M�ĵ�һ�������ɴ�С��˳����___________________(��Ԫ�ط��ű�ʾ)��
(3)�����й�����Ԫ�ص�˵���У���ȷ����______________________(�����)��
��G���ʵ��۵����J���ʣ�����ΪG���ʵĽ�������ǿ
��J��X���ã�����J��������Һ���û���X
�۽�J
��RE3�е����QE4����Ҫ����Ϊǰ����Է��������ϴ�
��һ��Q2E4�����к�������Ҽ���һ���м�
(4)���ô�����̨��̫�շ�����EQ9R����֪����������ԭ�Ӿ��γ�8���ӻ�2�����ȶ��ṹ����ֱ���η��ӣ���������λ����д����ṹʽ��_________________��
(5)G��R����ֱ�ӻ�������һ�����ӻ�����G3R���þ����������ʯī�IJ�״�ṹ��ÿ���У�Gԭ�ӹ���ƽ�������Σ�ÿ�������ε�������һ��Rԭ�ӡ������֮�仹����һ��������ԭ�ӡ�������Щ���ӵ�ԭ��Ӧ����____________(��G��R��Ԫ�ط���)��
(B)��ʵ�黯ѧ��
ij������ʾ����ʹ˫��ˮ�ֽ�Ĵ����кܶ��֣��������(������)�������ʹ���(��FeCl3)�������(��MnO2)�ȶ��ǽϺõĴ�����ijʵ��С��ͨ���ⶨ˫��ˮ�ֽ������O2��ѹǿ��̽���ֽ�����������Ѵ����Լ�̽����Ѵ������ʵĴ�������
(һ)̽��һ��
ʵ�鲽��
(1)����ƿ�м���50 mL 1.5����˫��ˮ
(2)�ֱ�����ƿ�м�
(3)�ɼ��ͼ�¼���ݡ�
(4)�������ݵó��±�
��ͬ������ѹǿ��ʱ��б�ʡ��ıȽ�
���� | ���� | ������ | �Ȼ�ͭ | �Ȼ��� | ����ͭ | �������� |
ѹǿ��ʱ���б�� | 0.191 87 | 0.002 42 | 0.007 93 | 0.030 5 | 0.015 47 | 1.833 6 |
�ٸá�̽��һ��ʵ���������_____________________________________________________��
�ڸ�ʵ�����ó��Ľ�����_______________________________________________________��
(��)̽�������������̴�����Ѵ�����
��ʵ��С���ͬѧ�ڽ���̽������ʵ��ʱ���õ���һϵ�е�ͼ�������ݡ��ο���ͼ�ͱ���ֱ�ش�������⡣
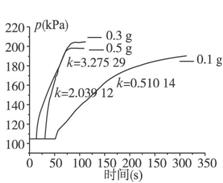
3%��˫��ˮ�벻ͬ�����������̵�ѹ����ʱ��ͼ
������ͬŨ�ȵ�˫��ˮ�ڲ�ͬ�����Ķ��������������ռ���ͬ״����ͬ���O2����ʱ��
MnO2 ʱ�� H2O2 | |||
1.5�� | 223 s | 67 s | 56 s |
3.0�� | 308 s | 109 s | 98 s |
4.5�� | 395 s | 149 s | 116 s |
����ͼ�������������ǿ��Եó���
��ͬŨ�ȵ�˫��ˮ�ķֽ��������Ŷ����������������Ӷ�_________________�������Ӧʱ��_______________��
�������ʵ�����ͽ�ʡҩƷ�ĽǶ��ۺϷ���������Ϊ������ѡ��3.0%��˫��ˮ������___________ g�Ķ���������ʹʵ��Ч����ѡ����жϵ�������______________________��
�ݸ�С���ijͬѧͨ���������ݵó��˵�����������ͬʱ˫��ˮ��Ũ��ԽС��Ӧ����Խ��Ľ��ۣ�����Ϊ�Ƿ���ȷ____________�����������________________________________��
��15�֣�����ѧ---ѡ��ģ�飺���ʽṹ�����ʡ�
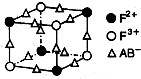
�±�Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
��ش��������⣺
��1����������d����Ԫ���� �����ţ���
��2��Ԫ�آ��γɵ���ۺ�����������幹����________��������ԭ�ӵ��ӻ����������_______��
��3��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ���� ��
| A�������к������ | B�����ڷǼ��Է��� |
| C������4���Ҽ���1���м� | D�����⻯������У���ԭ�Ӳ���sp2�ӻ� |
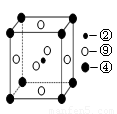
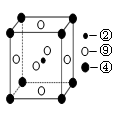
��5����ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ ���ö�Ӧ��Ԫ�ط��ű�ʾ����
 ����ѧ--ѡ��3���ʽṹ�����ʡ�
����ѧ--ѡ��3���ʽṹ�����ʡ�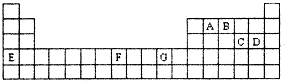
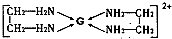 ���������к��еĻ�ѧ��������
���������к��еĻ�ѧ��������