��Ŀ����
��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L������Һ����������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚϲ���ȷ���� �� ��
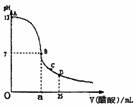
A����A��B����һ�㣨������A��B���㣩����Һ��һ�����У�c��Na+��>c(CH3COO�D )>c(OH�D )>c(H+)
B����B�㣬a>12.5�����У�c��Na+��=c(CH3COO�D)>c(OH�D)=(H+)
C����C�㣺c(CH3COO�D)> c��Na+��>(H+)>c(OH�D)
D����D�㣺c(CH3COO�D)+c(CH3COOH)=2 c��Na+��
A

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
 ��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L���ᣬ��������ͼ��ʾ���й�����������ǣ�������
��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L���ᣬ��������ͼ��ʾ���й�����������ǣ������� ��2011?ӥ̶��ģ�������£���25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ��pH ��μ� CH3COOH��Һ����Ĺ�ϵ������ͼ��ʾ������������Һ���ʱ������仯�������й�����Ũ�ȹ�ϵ��˵��������ǣ�������
��2011?ӥ̶��ģ�������£���25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ��pH ��μ� CH3COOH��Һ����Ĺ�ϵ������ͼ��ʾ������������Һ���ʱ������仯�������й�����Ũ�ȹ�ϵ��˵��������ǣ�������
 ��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ��ʾ��
��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ��ʾ��