��Ŀ����
ij�о���ѧϰС��Ϊ�ⶨij��þ3��һ5������þ�Ͻ𣨲�������Ԫ�أ���þ������������������������ֲ�ͬʵ�鷽������̽������������ǵ���ƻش��й����⡣
��̽��һ��ʵ�鷽����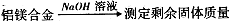 ��
��
�������ۣ���1��ʵ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2����ʵ���г�ȡ5.4g��þ�Ͻ��ĩ��Ʒ��Ͷ��VmL2.0mol��L NaOH��Һ�У���ַ�Ӧ����NaOH��Һ�����V�� mL��
��3��ʵ���У�����þ�Ͻ��ַ�Ӧ���ڳ���ʣ���������ǰ��������е�ʵ�������˳������Ϊ ��
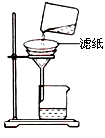
��̽������ʵ�鷽��������xg��þ�Ͻ��ĩ��������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�
�������ۣ���4��������Mg��������������ʵ���л���ⶨ�������� ��
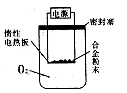
��5������ʵ���в��������Ϊyg����ԭ��þ�Ͻ��ĩ��þ����������Ϊ ���ú�x��y����ʽ��ʾ����
[̽����]ʵ�鷽����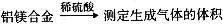 ��
��
�������ۣ���6��ͬѧ����ѡ���±ߵ�ʵ��װ�����ʵ�飬����Ϊ�����װ��������˳���ǣ�
a�� ������ӿ���ĸ��������һ��ȫѡ����
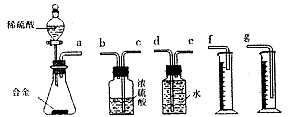
��7��ͬѧ����ϸ������6�������ӵ�ʵ��װ�ú������������ͼ��ʾ��ʵ��װ�á�
��װ���е���a�������� ��
��ʵ��ǰ���ʽ�ζ�����Һ������ֱ�������ͼ����������������Ϊ mL��
��������ͼװ����ȣ��ã�6�������ӵ�װ�ý���ʵ��ʱ��������������ԭ���� ����дһ�㣩��
��̽��һ��ʵ�鷽����
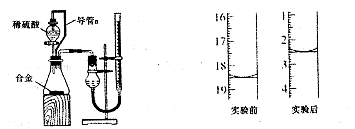
 ��
���������ۣ���1��ʵ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2����ʵ���г�ȡ5.4g��þ�Ͻ��ĩ��Ʒ��Ͷ��VmL2.0mol��L NaOH��Һ�У���ַ�Ӧ����NaOH��Һ�����V�� mL��
��3��ʵ���У�����þ�Ͻ��ַ�Ӧ���ڳ���ʣ���������ǰ��������е�ʵ�������˳������Ϊ ��
��̽������ʵ�鷽��������xg��þ�Ͻ��ĩ��������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�
�������ۣ���4��������Mg��������������ʵ���л���ⶨ�������� ��

��5������ʵ���в��������Ϊyg����ԭ��þ�Ͻ��ĩ��þ����������Ϊ ���ú�x��y����ʽ��ʾ����
[̽����]ʵ�鷽����
 ��
���������ۣ���6��ͬѧ����ѡ���±ߵ�ʵ��װ�����ʵ�飬����Ϊ�����װ��������˳���ǣ�
a�� ������ӿ���ĸ��������һ��ȫѡ����

��7��ͬѧ����ϸ������6�������ӵ�ʵ��װ�ú������������ͼ��ʾ��ʵ��װ�á�

��װ���е���a�������� ��
��ʵ��ǰ���ʽ�ζ�����Һ������ֱ�������ͼ����������������Ϊ mL��
��������ͼװ����ȣ��ã�6�������ӵ�װ�ý���ʵ��ʱ��������������ԭ���� ����дһ�㣩��
��1��2Al+2NaOH+2H2O=2NaAlO2+3H2��
��2��97
��3�����ˡ�ϴ�ӡ��������
��4�� ���պ���������
��5��
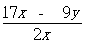
��6�� edg
��7����ʹ��Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����¡�
��16.00mL
��ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС��
���������
��1��þ����NaOH��Һ��Ӧ����������NaOH��Һ��Ӧ������2Al+2NaOH+2H2O=2NaAlO2+3H2��
��2������þ3��һ5������þ�Ͻ𣨲�������Ԫ�أ�������Mg����С����Ϊ5.4X3%="0.162g," ������������ 5.4-0.162=5.238g,n(Al)=5.238��27=0.194mol,���ݷ���ʽ��֪����ҪNaOH����97mL.
��3��ʵ���У�����þ�Ͻ��ַ�Ӧ���ڳ���ʣ���������ǰ��������е�ʵ�������˳������Ϊ���ˡ�ϴ�ӡ��������.
��4��Mg�����ֱ�����MgO��Al2O3,����Ҫ�������պ�����������
��5����MgΪnmol,AlΪm mol,�ж�Ԫһ�η�����⣬24n+27m=x��40n+51m=y,���
m(Mg)=
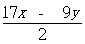 ,��þ����������Ϊ��
,��þ����������Ϊ��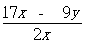
��6��ֻҪ�ܲ�����ˮ��������Ϳ�֪������������������������װ�����Ӿ����������ˮѹ����Ͳ�У�������Ͳ��ˮ��������������������ѡedg���ɡ�
��7���ٵ���a��������ͨ��ԭ����Ŀ����ʹ��Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����¡�
������ǰ��������18.50-2.50=16.00mL
��ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС��

��ϰ��ϵ�д�
�����Ŀ
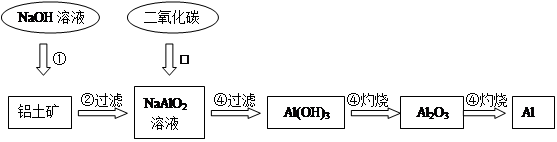

 4AlCl3+3O2
4AlCl3+3O2 AlCl3+X����Ϊȷ������X�Ƿ��ǻ�����壬ijͬѧ��X����ͨ�����ȵ�����ͭ�ͳ����ʯ��ˮ���ٸ��������жϡ��ò����Ƿ���ȷ��������ȷ������ȷ�����жϣ� ����˵������ ��
AlCl3+X����Ϊȷ������X�Ƿ��ǻ�����壬ijͬѧ��X����ͨ�����ȵ�����ͭ�ͳ����ʯ��ˮ���ٸ��������жϡ��ò����Ƿ���ȷ��������ȷ������ȷ�����жϣ� ����˵������ ��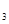 �����ڸ����ʵ�˵����ȷ���ǣ� ��
�����ڸ����ʵ�˵����ȷ���ǣ� ��