��Ŀ����
ijʵ��С����ʵ��������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL30%��KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8%��NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ��
����д���пհף�
(1)������ʦ��ʾ��װ�����һ�����ƣ�
��.Ӧ�ڢ����֮�䰲װʢ��(���Լ�����)______________�ľ���װ�ã�
��.��������һװ�ã��뻭�����Ӳ��ֵ�װ��ʾ��ͼ����ע����ʢ�Լ����ơ�
(2)д������Cl2��KOH��Һ��Ӧ�Ļ�ѧ����ʽ(������ƽ)______________��
(3)�ܵ��Թ�����Һ����ɫӦ�ȱ�Ϊ______________ɫ�������Ϊ��ɫ���ִ���ɫ��Ϊ______________ɫ��
(4)ʵ�����ʱ��Ӧ��Ϩ��______________���ľƾ��ƣ��Ԣٵ���ƿ�вд�����Ĵ���������______________��
(5)84����Һ����Ч�ɷ���NaClO��������Һ������ɱ������������ʹ��ɫ������ɫ����ԭ����______________(�û�ѧ����ʽ��ʾ)��
(1)��ʳ��ˮ ��(����ͼ)

(2)Cl2+KOH![]() KCl+KClO3+H2O
KCl+KClO3+H2O
(3)�� ������ɫ
(4)�� ��ȴ����ƿ����Ѹ������ƿ�м���һ������ϡNaOH��Һ���ٸ���ƿ����ҡ����ƿ��ַ�Ӧ
(5)NaClO+CO2+H2O![]() NaHCO3+HClO(���������𰸾���)
NaHCO3+HClO(���������𰸾���)
������(1)���Ƶõ�Cl2�л���HCl��Ӧ�ڢ١���֮�䰲װʢ�б���ʳ��ˮ�ľ���װ�ã���ȥHCl����Cl2�ж�������ֱ���ŷţ�Ӧ�ü�Һ���ա�
�����ͼ��
(2)Cl2+KOH![]() KCl+KClO3+H2O
KCl+KClO3+H2O
(3)��ɫʯ����Һ�ȱ��ɫ�������Ϊ��ɫ���ִ���ɫ��Ϊ������ɫ��
(4)Ϊ��ֹҺ�嵹������Ϩ��ڣ���������Ĵ�����������ȴ����ƿ����Ѹ������ƿ�м���һ����ϡNaOH��Һ���ٸ���ƿ�ǣ�ҡ����ƿʹ֮��ַ�Ӧ��

 ��У����ϵ�д�
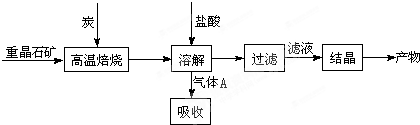
��У����ϵ�д�ij�о�С����ʵ��������Ʊ�Na2S2O3?5H2O�������£�
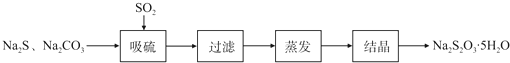
��1������װ����ͼ��ʾ��
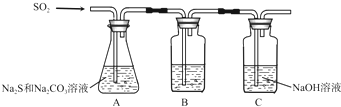
��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ���
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��飮������ʱCaCO3������Һ��pH=10.2��
��ѡ�Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ��Ca��NO3��2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ι�
| ��� | ʵ����� | Ԥ������ | ���� |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬ |
��Ʒ��NaCl | |
| �� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬ |
��Ʒ��NaOH |
��֪��Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O 2S2O32-+I2�TS4O62-+2I-��