��Ŀ����
NAΪ�����ӵ����������и��������У���ȷ����
��0.2 mol H2O2��ȫ�ֽ�ת�Ƶĵ�����Ϊ0.4NA
��25 �桢101 kPa�£�16 g O3��O2��������к��е���ԭ����ΪNA
��0.1 mol FeCl3�����ˮ�γɵĽ������ӵ���ĿΪ0.1 NA
��1 mol N2��3 mol H2��һ�������µ��ܱ������г�ַ�Ӧ�������ڵķ���������2NA
��0.2 mol H2O2��ȫ�ֽ�ת�Ƶĵ�����Ϊ0.4NA
��25 �桢101 kPa�£�16 g O3��O2��������к��е���ԭ����ΪNA
��0.1 mol FeCl3�����ˮ�γɵĽ������ӵ���ĿΪ0.1 NA
��1 mol N2��3 mol H2��һ�������µ��ܱ������г�ַ�Ӧ�������ڵķ���������2NA
| A���٢ڢ� | B���٢ڢ� | C���٢ڢۢ� | D���ڢ� |
D
����������ٷֽⷴӦΪ��2H2O2 = 2H2O + O2��H2O2����Ԫ�ػ��ϼ�Ϊ�C1�ۣ���ÿĦ��H2O2�ֽ�ʱת��1mol���ӣ�0. 2 mol H2O2�ֽ�ת�Ƶĵ�����ӦΪ0.2NA������
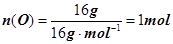 ������ԭ����ΪNA����ȷ���۽����������ɸ������������Ӿۼ���һ���γɵģ�Fe3+ˮ��Ҳ�Dz���ȫ�ģ�����0.1mol FeCl3�����ˮ�γɵĽ�����������0.1NA������N2��H2�ķ�ӦΪ���淴Ӧ��1 mol N2��3 mol H2���ܱ������в�����ȫת��ΪNH3������������������ʵ�������2mol��������������2NA����ȷ����ѡD��
������ԭ����ΪNA����ȷ���۽����������ɸ������������Ӿۼ���һ���γɵģ�Fe3+ˮ��Ҳ�Dz���ȫ�ģ�����0.1mol FeCl3�����ˮ�γɵĽ�����������0.1NA������N2��H2�ķ�ӦΪ���淴Ӧ��1 mol N2��3 mol H2���ܱ������в�����ȫת��ΪNH3������������������ʵ�������2mol��������������2NA����ȷ����ѡD��
��ϰ��ϵ�д�
�����Ŀ

