��Ŀ����
���Ͽ�ѧ�ǽ������뻯ѧ�йصĿ�ѧ�о����ص㣬ij�������ǽ�������K�����ַǽ���Ԫ����ɣ�����һ�ֳ�Ӳ���ʣ�������ĥ����ʴ�������ȳ���������������ԣ���������ѧ��ѧ�еij�������Ϊԭ���������ġ���ͼ��ʾΪ���������̣��������߿���ת����Ϊ̽��C����ɶ��衣��֪A��B��Ϊ�ǽ������ʣ�G��F��H��������ˮ��Ϊ��ɫ��ĩ��ͼ�г�M��K������AԪ�أ����Ϊ��ѧ��ѧ�������ʣ�
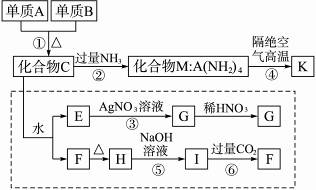
������������⡣
��1��ָ��K���������ľ�������_________��
��2��д����ѧʽ������B_________��������F_________��
��3��д�����з�Ӧ�����ӷ���ʽ��
��Ӧ��___________________________;
��Ӧ��___________________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ___________________________��

������������⡣
��1��ָ��K���������ľ�������_________��
��2��д����ѧʽ������B_________��������F_________��
��3��д�����з�Ӧ�����ӷ���ʽ��
��Ӧ��___________________________;
��Ӧ��___________________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ___________________________��
(1)ԭ�Ӿ���
(2)Cl2 H2SiO3(��H4SiO4)
(3)Cl-+Ag+ AgCl�� SiO2+2OH-
AgCl�� SiO2+2OH-
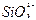 +H2O
+H2O
(4)3Si(NH2)4 Si3N4+8NH3��
Si3N4+8NH3��
(2)Cl2 H2SiO3(��H4SiO4)
(3)Cl-+Ag+
 AgCl�� SiO2+2OH-
AgCl�� SiO2+2OH-
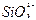 +H2O
+H2O(4)3Si(NH2)4
 Si3N4+8NH3��
Si3N4+8NH3�� ���ݻ�����M��A���γ��ĸ��ۼ�֪��AԪ��Ϊ�ļ�Ԫ�أ������߿�����һ֧��֪E����Cl-������һ֧��֪F�к�AlԪ�ػ�SiԪ�أ���ϵ���ϼ�֪F�к���Ԫ��(��Ŀ��Ҳ˵����Ϊ�ǽ���Ԫ��)����CΪSiCl4����SiCl4+3H2O 4HCl+H2SiO3������E��HCl��F��H2SiO3��
4HCl+H2SiO3������E��HCl��F��H2SiO3��
 4HCl+H2SiO3������E��HCl��F��H2SiO3��
4HCl+H2SiO3������E��HCl��F��H2SiO3��
��ϰ��ϵ�д�
�����Ŀ
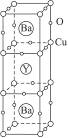
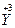 ��
��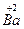 ��
��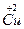 ��
��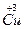 ���Լ���Cu�ڻ������е�ƽ�����ϼ�Ϊ______________�����ּ�̬Cu��ԭ�Ӹ���֮��Ϊ____________��
���Լ���Cu�ڻ������е�ƽ�����ϼ�Ϊ______________�����ּ�̬Cu��ԭ�Ӹ���֮��Ϊ____________��