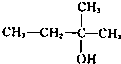
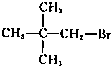
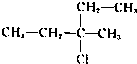��Ŀ����
������Һ�У���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A������NH4+��Cl-��H+��OH-���ӵ���Һ�У�������Ũ��һ���ǣ�c��Cl-����c��NH4+����c��H+����c��OH-�� |
| B��pH=8�İ�ˮ���Ȼ�淋Ļ����Һ�У�c��Cl-����c��NH4+�� |
| C��0.1mol/L��Na2S��Һ�У�c��OH-��=c��H+��+c��HS-��+2c��H2S�� |
| D��pH=3��һԪ���pH=11��һԪǿ��������Ϻ����Һ�У�c��OH-��=c��H+�� |
A������NH4+��Cl-��H+��OH-���ӵ���Һ�У�����Ϊһˮ�ϰ����Ȼ�淋Ļ����Һ������ܴ���c��NH4+����c��Cl-����c��OH-����c��H+������A����
B��pH=8�İ�ˮ���Ȼ�淋Ļ����Һ���Լ��ԣ��ɵ���غ�c��NH4+��+c��H+��=c��Cl-��+c��OH-����֪��c��Cl-����c��NH4+������B����
C���������غ��֪��c��OH-��=c��H+��+c��HS-��+2c��H2S������C��ȷ��
D��һԪ�ᣬΪǿ������ᣬ��pH=3��һԪ���pH=11��һԪǿ��������Ϻ����Һ�У�c��OH-����c��H+������D����
��ѡC��
B��pH=8�İ�ˮ���Ȼ�淋Ļ����Һ���Լ��ԣ��ɵ���غ�c��NH4+��+c��H+��=c��Cl-��+c��OH-����֪��c��Cl-����c��NH4+������B����
C���������غ��֪��c��OH-��=c��H+��+c��HS-��+2c��H2S������C��ȷ��
D��һԪ�ᣬΪǿ������ᣬ��pH=3��һԪ���pH=11��һԪǿ��������Ϻ����Һ�У�c��OH-����c��H+������D����
��ѡC��

��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
 )��c(Cl��)��c(H��)��c(OH��)
)��c(Cl��)��c(H��)��c(OH��)