��Ŀ����
(16��)ֱ������̼-̼���ķ�Ӧ��ʵ�ָ�Ч����ɫ�л��ϳɵ���Ҫ;������������ż����Ӧ�ǽ��걶�ܹ�ע��һ��ֱ������̼-̼���������ͷ�Ӧ�����磺
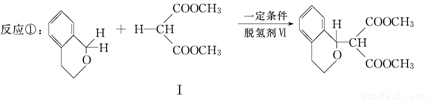
�������������ºϳ�·��ã�
��(����ʽΪC3H8O2)������
(1)�������ķ���ʽΪ ��
����ȫˮ��Ļ�ѧ����ʽΪ (ע������)��
(2)������II�������ᷴӦ�Ļ�ѧ����ʽΪ (ע������)��
(3)�������û�����ԣ���ṹ��ʽΪ �����һ��ͬ���칹������뱥�� NaHCO3 ��Һ��Ӧ�ų�CO2����������Ľṹ��ʽΪ ��
(4)��Ӧ����1���������(�ṹ��ʽ��ͼ)���ӻ��2����ԭ�Ӻ�ת���1�������廯������ӡ��÷����廯������ӵĽṹ��ʽΪ________��
(5)1 ���� ��
1 ����
��
1 ����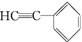 ��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��
��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��
��1��C5H8O4
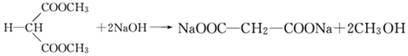
(2)CH2OH-CH2-CH2OH+2HBr CH2Br-CH2-CH2Br+2H2O
CH2Br-CH2-CH2Br+2H2O
(3)OHC-CH2-CHO CH2=CH-COOH
(4) 
(5)  8
8
�����������⿼�����л�������Ľṹ�����ʣ��ѵ��Ƕ����Ѹ���Ϣ��Ǩ��������
��1��������I���ڶ������ɷ���ˮ�ⷴӦ��
��2���ۺϷ����ж�IIΪ��CH2OH-CH2-CH2OH�������������������ᷢ��ȡ����Ӧ���ɶ�±����
��3��II����Ϊ��ԪȩIII��OHC-CH2-CHO����һ��ͬ���칹������뱥�� NaHCO3 ��Һ��Ӧ�ų�CO2�������С�COOH���ٿ��ǵ������Ͷȿ�֪VΪ��CH2=CH-COOH
��4���������������C=O�Ͽ��ֱ���Hԭ�Ӽӳ�Ϊ��OH����Ԫ��ת��Ϊ������
��5��ǰ�ߡ�CH3�е�C��H�Ͽ������ߡ�C��CH�е�C��H�Ͽ������е����ӵ�����̼ԭ���γ��¼����õ��� �����б�����̼̼���������������ӳɡ�
�����б�����̼̼���������������ӳɡ�

(16��)̼������������������Ҫ�ķǽ���Ԫ�ء�
��1��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)����H����ֱ�Ӳ�����ԭ����?? ??????????? ��
��֪��a��2CO(g)+O2(g)=2CO2(g)??? ��H =-566��0 kJ��mol-1
b��CH4(g)+2O2(g)=CO2(g)+2H2O(g)?? ��H =-890��0 kJ��mol-1
��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)���Ȼ�ѧ����ʽΪ ??????????????????????????????????? ��
��2����ҵ�Ϻϳɰ����ķ�ӦΪ��N2(g)+3H2(g) 2NH3(g)? ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ���������N2��ƽ��ת����(
2NH3(g)? ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ���������N2��ƽ��ת����( )����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
)����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺

����Ӧ�ﵽƽ��״̬Bʱ���������ݻ�10 L����T1ʱ���ϳɰ���Ӧ��ƽ�ⳣ��K= ???? L2��mol-1��
��ƽ��״̬��A�䵽Cʱ����Ӧ��ƽ�ⳣ��K(A)??? K(C)(����>������<������=��)��
��3����25��ʱ��HSCN��HClO��H2CO3�ĵ��볣�����±���
HClO | HSCN | H2CO3 |
K=3.210-8 | K=0.13 | Kl=4.210-7 K2=5.610-11 |
��1 mol��L-1��KSCN��Һ�У��������ӵ�Ũ���ɴ�С��˳��Ϊ? ??? >????? > ??? > ???????? ��
����Na2CO3��Һ�м������HClO��Һ����Ӧ�Ļ�ѧ����ʽΪ ??????? ? ��
��25��ʱ��Ϊ֤��HClOΪ���ᣬijѧϰС���ͬѧû������������ʵ�鷽�����������ַ����У�����Ϊ�ܹ��ﵽʵ��Ŀ�ĵ���??? (�����и��������)��
a����pH�Ʋ���0��1 mol��L-1NaClO��Һ��pH�������pH>7����֤��HClOΪ����
b����pH��ֽ����0��01 mol��L-1HClO��Һ��pH�������pH>2����֤��HClOΪ����
c������������Ũ�Ⱦ�Ϊ0��1 mol��L-1��HClO��Һ������ĵ����ԣ������HClO��Һ�ĵ������������ᣬ��֤��HClOΪ����



 �� 1 ����
�� 1 ���� ��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��
��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��
 +HCl
+HCl
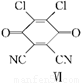
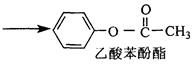
 ���������ȡ�����ײ�������Ժá�����С���ص㡣��֪�����ӷ���������ǻ�ֱ��������̼ԭ�ӵ��ڡ���λ�ϵ���ԭ���кܺõķ�Ӧ���ԣ���ijЩ���ʻ��Ļ�����R��C O��R�䣨R��R����������Hԭ�ӣ����������Ϸ�Ӧ�����µ��л����ˮ�����ӻ��ܷ������·�Ӧ�����л�������
���������ȡ�����ײ�������Ժá�����С���ص㡣��֪�����ӷ���������ǻ�ֱ��������̼ԭ�ӵ��ڡ���λ�ϵ���ԭ���кܺõķ�Ӧ���ԣ���ijЩ���ʻ��Ļ�����R��C O��R�䣨R��R����������Hԭ�ӣ����������Ϸ�Ӧ�����µ��л����ˮ�����ӻ��ܷ������·�Ӧ�����л�������
 +HCl
+HCl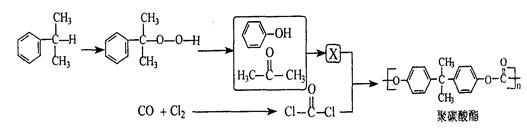
 mol��
mol�� ��
��