��Ŀ����
A�ǵ���������Ԫ����ɵĻ����������������ˮ֮���ڽṹ�ϵĹ�ϵ���ơ�A�����е������������Ϊ7/1��������������⡣��1��A�Ļ�ѧʽ��_________________________��
��2��A��ˮ��Һ��____________����ᡱ������С����ԣ�1 mol A������____________mol HCl(��NaOH)�����кͷ�Ӧ�γ����Σ�д���γɵ����к��е�ԭ�ӵ����ӵĵ���ʽ��____________________��
��3��A��������ԭ��Ӧ��Ҳ������������ƣ��ȿ������������ֿ�����ԭ��������ݼ�̬�����������������ԭ��___________________________________��
��1��N2H4
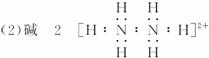
(3)N2H4��N�Ļ��ϼ�Ϊ-2����H2O2��O��-1��һ����N�Ļ��ϼۿ����ɽ����ڻ�ѧ�仯�мȿ�����ԭ����Ҳ����������
��������2���ɣ�1����֪A�Ļ�ѧʽΪN2H4�������ʽΪ![]() ,ÿ��Nԭ����һ�Թ¶Ե��ӣ��ɷֱ���1��H+�γ���λ������1 mol A������2 mol HCl�����кͷ�Ӧ��
,ÿ��Nԭ����һ�Թ¶Ե��ӣ��ɷֱ���1��H+�γ���λ������1 mol A������2 mol HCl�����кͷ�Ӧ��

 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| |||||||||||||||