��Ŀ����
�����ڵ�����Ԫ��X��Y��Z��W��ԭ��ϵ����������Zԭ��������������X��Y��W����ԭ�ӵ�����������֮�ͣ�Z��X��Y��W����Ԫ���γ�ԭ�Ӹ���֮��Ϊ1��1�Ļ�����ֱ���A��B��C�����л�����C�ڿ��������ױ��ʣ���ش�
��1��д��Z��ԭ�ӽṹʾ��ͼ______________________________��
��2��д��������YZ2����ʽ��______________�ռ乹��Ϊ��_______________��д��������C�ĵ���ʽ��_____________��ѧ�������У�_____________��
��3��д��������C�ڿ����б��ʵĻ�ѧ����ʽ��____________________
��1��д��Z��ԭ�ӽṹʾ��ͼ______________________________��
��2��д��������YZ2����ʽ��______________�ռ乹��Ϊ��_______________��д��������C�ĵ���ʽ��_____________��ѧ�������У�_____________��
��3��д��������C�ڿ����б��ʵĻ�ѧ����ʽ��____________________
(1)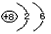 ;
;
(2)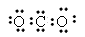 ��ֱ���� ��
��ֱ���� ��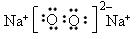 �����Ӽ����ۼ�
�����Ӽ����ۼ�
(3)2Na2O2 + 2CO2 === 2Na2CO3 + O2 ��2Na2O2 + 2H2O === 4NaOH + O2��
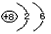 ;
;(2)
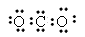 ��ֱ���� ��
��ֱ���� ��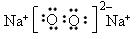 �����Ӽ����ۼ�
�����Ӽ����ۼ�(3)2Na2O2 + 2CO2 === 2Na2CO3 + O2 ��2Na2O2 + 2H2O === 4NaOH + O2��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
