��Ŀ����
ʵ������ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
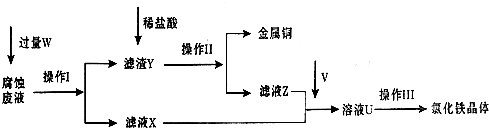
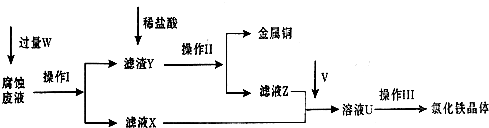
I��������������̣�Բ���������⣺
��1������I���õ��IJ����������ձ����������⣬��������______��______�����������ƣ�
��2������������ȣ���ȴ�����£�����ƽ����������Ϊb1 g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1-b2______������Ϊ���ճ�֣�������Ʒ����Ҫ�������оƾ��ơ����żܡ�______��______��______������ǯ��
��3����������������W1g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������______��
II����ͬѧ����������Բ������·������ⶨ��
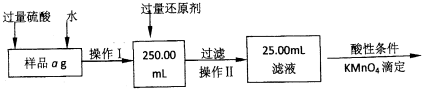
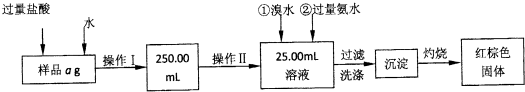
��4������ȡ����Һ�͵ζ���������Ҫ�õ��ĵζ�����______����ţ�
A��һ֧��ʽ�ζ��ܺ�һ֧��ʽ�ζ��ܡ���B��֧��ʽ�ζ��ܡ���C��֧��ʽ�ζ���
��5���ζ��յ������Ϊ______��
��6�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص�����������______����֪����������MnO4-����ԭΪMn2+��
��7������������У����в������������Ԫ�ص���������ƫ�ߵ���______����ţ�
A���ܽ���Ʒʱ��ϡ�������ϡ����
B��������������ԭ��
C����ȡ��Һʱδ��ϴ��Ӧ�ζ���
D���ζ�ǰ����ʱ�������������ݣ��ζ��������ݲ����ڵζ��ܼ��
E������c mol/L KMnO4����Һʱδ��ϴ��Һһ��ת��������ƿ
F������ʱ����ƿ�з���25.5mL����Fe2+�Ĵ���Һ��
��2��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g������������Ҫ�����������ǣ����������ʴ�Ϊ��С��0.1g�������������ǣ���������
��3������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��
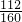 ����Ʒ����Ԫ�ص�����������
����Ʒ����Ԫ�ص�����������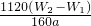 ��100%��
��100%���ʴ�Ϊ��
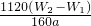 ��100%��
��100%����4����ȡ����Һ�͵ζ���������Ҫ��ʽ�ζ��ܵζ�������װ��Һ���������Һ����ȡ����Һ�������Ӻ������ӵ���Һ�����ԣ���Ҫ��֧��ʽ�ζ��ܣ���ѡB��
��5���ζ��յ������ø��������Һ���Ϻ�ɫ��ָʾ��Ӧ�յ㣬����Ϊ���������һ����Һʱ����ƿ����Һ��ɫ��Ϊ�Ϻ�ɫ�Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����������һ����Һʱ����ƿ����Һ��ɫ��Ϊ�Ϻ�ɫ�Ұ�����ڲ���ɫ��
��6������Ԫ����������ΪX%�����ݷ�Ӧ5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O��
5Fe2+��5Fe3+��KMnO4
5��56 1
a��X%��
 c��b��10-3
c��b��10-3��Ԫ�ص�����������X%=
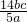 ���ʴ�Ϊ��
���ʴ�Ϊ��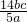 ��
����7��A���ܽ���Ʒʱ��ϡ�������ϡ���ᣬ������ؾ���ǿ�����ԣ��������ᣬ��Һ�е������ӻᱻ�����������ĸ�����أ�����ʵ��ⶨ�����ƫ�ߣ�
B��������������ԭ���������������������������������ı�Һ�����ƫ�ߣ�
C����ȡ��Һʱδ��ϴ��Ӧ�ζ��ܣ�����ҺŨ�ȼ�С���ⶨ���ƫС��
D���ζ�ǰ����ʱ�������������ݣ��ζ��������ݲ����ڵζ��ܼ�ˣ������ı�Һ�����С�����ƫС��
E������c mol/L KMnO4����Һʱδ��ϴ��Һһ��ת��������ƿ�����Ƶĸ��������ҺŨ��ƫС���ζ�ʱ���ĸ��������Һ��������ƫ�ߣ�
F������ʱ����ƿ�з���25.5mL����Fe2+�Ĵ���Һ��Ũ�Ȳ��䣬��Ӱ�죻
��ѡABE��
��������1������I��������Һ250.00ml�����ݲ�������������õ��IJ����������ձ����������⣬����250ml����ƿ�ͽ�ͷ�ιܣ�
��2��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g������������Ҫ�������н��м��ȣ�����������ʹ�÷����Ͳ���ѡ��������
��3��������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����
��4����ȡ����Һ�͵ζ���������Ҫ��ʽ�ζ��ܵζ�������װ��Һ���������Һ����ȡ����Һ�������Ӻ������ӵ���Һ�����ԣ���Ҫ��֧��ʽ�ζ��ܣ�
��5���ζ��յ������ø��������Һ���Ϻ�ɫ��ָʾ��Ӧ�յ㣬����Ϊ���������һ����Һʱ����ƿ����Һ��ɫ��Ϊ�Ϻ�ɫ�Ұ�����ڲ���ɫ
��6�����ݸ�����غ��������ӵ�������ԭ��Ӧ������ϵ���㣻
��7�����в������������Ԫ�ص���������ƫ�ߵ�ѡ�����Ϊ��
A���ܽ���Ʒʱ��ϡ�������ϡ���ᣬ���������Һ�������������ӣ����ı�Һ����ࣻ
B��������������ԭ���������������ӣ�
C����ȡ��Һʱδ��ϴ��Ӧ�ζ���ϡ�ͱ�Һ���ζ������������������
D���ζ�ǰ����ʱ�������������ݣ��ζ��������ݲ����ڵζ��ܼ�ˣ������ı�Һ�����С��
E������c mol/L KMnO4����Һʱδ��ϴ��Һһ��ת��������ƿ�����Ƶĸ��������ҺŨ��ƫС���ζ�ʱ���ĸ��������Һ�������
F������ʱ����ƿ�з���25.5mL����Fe2+�Ĵ���Һ����Ӱ��ⶨ��ҺŨ�ȣ�
���������⿼������Һ���Ƶķ����Ͳ��裬����ѡ�������յ�����ѡ��Ͳ��裬�ζ�ʵ��Ĺ��̷����жϣ�������Ӧ�ã���Ŀ�Ѷ��еȣ�

��.��ˮ���Ȼ�����Һ����ѧ��ѧʵ���еij����Լ���Ũ�Ƚ�Сʱ����Һ���ʻ�ɫ��ijͬѧ��ϡ��ˮ��ϡFeCl2��Һ��ϣ�������Һ�Ի�ɫ��Ϊ̽����ˮ��FeCl2��Һ����ܷ�����Ӧ����ͬѧ���������ʵ�鷽����
| ���� | ʵ������ | ʵ����� | |
| ����1 | ȡ���������Һ������NaOH��Һ���� | �õ����ɫ���� | �����˻�ѧ��Ӧ |
| ����2 | ȡ���������Һ���������Ȼ�̼��Һ���� | �л���ʳȻ�ɫ | δ������ѧ��Ӧ |
| ����3 | ȡ���������Һ��������۵⻯����Һ���� | ��Һ����ɫ | δ������ѧ��Ӧ |
��ش��������⣺
��1������2�Ľ��۲���������������
��2������3�Ľ������Բ����������ܷ�����Ӧ�����ӷ���ʽ
��3�����������һ����ʵ�鷽�������������������������ۡ������ж���ˮ��FeCl2��Һ�Ƿ�Ӧ��
��
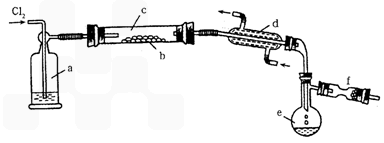
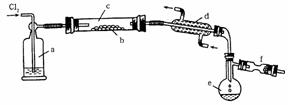 ��.S2Cl2��һ���ӷ���Һ�壨�۵㣺��76�棬�е㣺138�棩��������ˮ����ˮ�ⷴӦ����������H2S��SO2��H2SO3��H2SO4�����ʡ����������������ڵ�����ͨ��������������S2Cl2����ͼ��ʵ������S��Cl2�Ʊ�S2Cl2��װ�ã��г�װ�á�����װ�þ�����ȥ����
��.S2Cl2��һ���ӷ���Һ�壨�۵㣺��76�棬�е㣺138�棩��������ˮ����ˮ�ⷴӦ����������H2S��SO2��H2SO3��H2SO4�����ʡ����������������ڵ�����ͨ��������������S2Cl2����ͼ��ʵ������S��Cl2�Ʊ�S2Cl2��װ�ã��г�װ�á�����װ�þ�����ȥ����
��1��װ��a��Ӧ���Լ�Ϊ__________��װ��d��������_________������������_________��
��2����ʵ��IJ���˳��ӦΪ__________������ű�ʾ����
�ټ���װ��c ��ͨ��Cl2 ��ͨ����ˮ ��ֹͣͨCl2 ��ֹͣ����װ��c
��3����S2Cl2��ˮ���������ͨ����ˮ�У����۲쵽_________���������֤��ˮ����������������ɡ�