��Ŀ����
��14�֣�ijͬѧ������ͼװ���Ʊ���������������a�Թ�����Ũ���ᡢ�Ҵ�������Ļ��Һ�� b�Թ�ʢ����Na2CO3��Һ���ش��������⣺
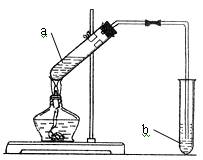
��1��ָ��װ����һ�����ԵĴ���
��2������Ũ���ᡢ�Ҵ�������Ļ��Һ����ȷ����˳���� ��
��3��д���Թ�a����������Ӧ�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��4����Ӧ��������b�ԹܵĻ��Һ���ɹ۲쵽b�Թ�����ϸС����ð����д����ʾ�÷�Ӧ�����ӷ���ʽ ��
��5����b�Թ��з�������������ķ��뷽���� ��
��6��Ũ�����ڴ˷�Ӧ�е������� ��

��1��ָ��װ����һ�����ԵĴ���
��2������Ũ���ᡢ�Ҵ�������Ļ��Һ����ȷ����˳���� ��
��3��д���Թ�a����������Ӧ�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��4����Ӧ��������b�ԹܵĻ��Һ���ɹ۲쵽b�Թ�����ϸС����ð����д����ʾ�÷�Ӧ�����ӷ���ʽ ��
��5����b�Թ��з�������������ķ��뷽���� ��
��6��Ũ�����ڴ˷�Ӧ�е������� ��
��14��,ÿ��2�֣�
��1��b�Թ��е�������Һ���£�2�֣�
��2���ȼ��Ҵ��ٵμ�ŨH2SO4���ӱ����������ᣬ��;��ȣ�2�֣�
��3��CH3CH2OH +CH3COOH CH3COOCH2CH3 + H2O ��2�֣�
CH3COOCH2CH3 + H2O ��2�֣�
������Ӧ ��2�֣�
��4��2CH3COOH+CO32����2CH3COO�� +CO2��+H2O��2�֣�
��5����Һ��2�֣� ��6����������ˮ������2�֣�
��1��b�Թ��е�������Һ���£�2�֣�
��2���ȼ��Ҵ��ٵμ�ŨH2SO4���ӱ����������ᣬ��;��ȣ�2�֣�
��3��CH3CH2OH +CH3COOH
 CH3COOCH2CH3 + H2O ��2�֣�
CH3COOCH2CH3 + H2O ��2�֣�������Ӧ ��2�֣�
��4��2CH3COOH+CO32����2CH3COO�� +CO2��+H2O��2�֣�
��5����Һ��2�֣� ��6����������ˮ������2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 ���Ʋ��ҩ�ﲻ���ܾ��еĻ�ѧ������ �� ��
���Ʋ��ҩ�ﲻ���ܾ��еĻ�ѧ������ �� �� Һ������ɫ��Ӧ
Һ������ɫ��Ӧ