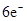��Ŀ����
2011��4��27��ij�й��ֲ̾��400�˴�װ�����겹��Ӫ���̷�1 401���������̷۱�����Ϊ�����������β������߳�����ֵ7.8��������ʳ�ÿ����°�����֪NaNO2�ܷ������·�Ӧ��2NaNO2��4HI===2NO��I2��2NaI��2H2O��
(1)������Ӧ����������________������0.75 mol�Ļ�ԭ������������ԭ����������________ mol��
(2)����������Ӧ����������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������Т�����ˮ���ڵ��۵⻯����ֽ���۵��ۣ��ܰ��ǣ���ʳ�ף��ްơ�����ʵ��ʱ������ѡ�õ�������__________��
(3)ij��������Һ�У���2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ�������Լ���________(�����)��ʹNaNO2ת��Ϊ�����������Ⱦ��N2����Ӧ�Ļ�ѧ����ʽΪ_____________________________________(���������ת�Ƶ���Ŀ�ͷ���)��
��NaCl����NH4Cl����H2O2����ŨH2SO4
(1)������Ӧ����������________������0.75 mol�Ļ�ԭ������������ԭ����������________ mol��
(2)����������Ӧ����������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������Т�����ˮ���ڵ��۵⻯����ֽ���۵��ۣ��ܰ��ǣ���ʳ�ף��ްơ�����ʵ��ʱ������ѡ�õ�������__________��
(3)ij��������Һ�У���2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ�������Լ���________(�����)��ʹNaNO2ת��Ϊ�����������Ⱦ��N2����Ӧ�Ļ�ѧ����ʽΪ_____________________________________(���������ת�Ƶ���Ŀ�ͷ���)��
��NaCl����NH4Cl����H2O2����ŨH2SO4
��1��NaNO2 0.75 ��2���� �� ��3���� 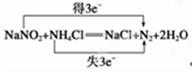

��1�����ݷ���ʽ��֪�����������е�ԭ�ӵĻ��ϼ۴ӣ�3�۽��͵���2�ۣ��õ����ӣ�����ԭ��ʣ����������0.75mol�⻯��ʧȥ0.75mol�����Ը��ݵ��ӵĵ�ʧ�غ��֪������ԭ��������Ҳ��0.75mol��
��2���������ƾ��������ԣ��������⻯�أ����Ȼ��Ʋ��ܡ����÷�Ӧ������������С���еģ����Դ�ѡ�ڢݡ�
��4����������ת��Ϊ������˵��������������������������Ҫ���뻹ԭ���Ȼ�泥���Ӧ�ķ���ʽ���𰸡�
��2���������ƾ��������ԣ��������⻯�أ����Ȼ��Ʋ��ܡ����÷�Ӧ������������С���еģ����Դ�ѡ�ڢݡ�
��4����������ת��Ϊ������˵��������������������������Ҫ���뻹ԭ���Ȼ�泥���Ӧ�ķ���ʽ���𰸡�

��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ

 Al2O3 +2Fe����ʵ��װ�ã��йظ÷�Ӧ������˵������ȷ����
Al2O3 +2Fe����ʵ��װ�ã��йظ÷�Ӧ������˵������ȷ����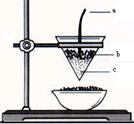
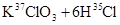

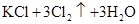 ���й������У���ȷ���ǣ� ��
���й������У���ȷ���ǣ� ��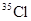
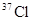
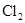 ����Է�����������71
����Է�����������71