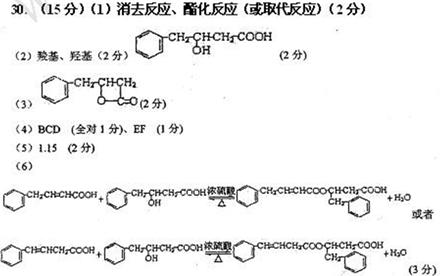
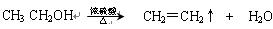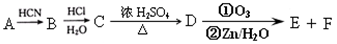��Ŀ����
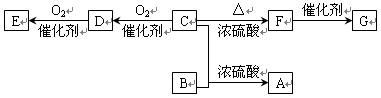
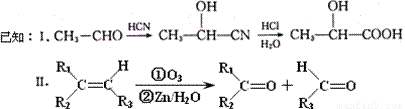
���֣���֪�л���A�����к��б�����ֻ��һ��������C�г��������һ����Ԫ�����л���A����Է�������M������200��������Ԫ�ص���������Ϊ26.7%����ȫȼ��ֻ����ˮk*s#5^u�Ͷ�����̼����֮�йص��л���ת����ϵ���£���ע�⣺���ַ�Ӧ����ʡ�ԣ�

��1����~�������漰�ķ�Ӧ����Ϊ ��
��2�� �л���A�к��������ŵ�����Ϊ________________��A�Ľṹ��ʽΪ____________��
��3���л���õĽṹ��ʽΪ____________________________
��4���л���A��F�л�Ϊͬ���칹�����____________��______________����д��ĸA��B��������
��5��16.2g�л���B��C��D��ɵĻ������ȫȼ�գ�����O2�����ʵ���Ϊ mol��6��д��A+D��E�Ļ�ѧ����ʽ_____________________________��
����:
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
(10��)��֪�л���A�Ľṹ��ʽΪ�� ���ش��������⣺
���ش��������⣺
��1��A�����������ŵ�����Ϊ�� ��
���и����ʷֱ���A������Ȼ�ϣ���ֻҪ�����ʵ������䣬��������������Ҳ������� ������ĸ���ţ�
| A��C2H6O | B��C3H4O2 | C��C2H4 | D��C3H6 |
��3����һ�������£�A���ܷ�Ӧ����һ�ָ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ��
��4����һ�������£�A�ܷ�Ӧ����B��B��ʹ������Ȼ�̼��Һ��ɫ��B���ܷ�Ӧ���ɸ߷��ӻ�����C,��д����B����C�Ļ�ѧ����ʽ��
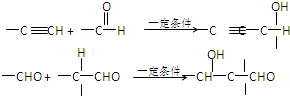



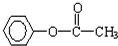 ��
��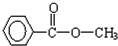 ��
��