��Ŀ����
ij�����ų��ķ�ˮ�к��д�����Fe2+��Cu2+��SO42-��ijУ�о���ѧϰС�������ͼ�����Ի���ͭ������������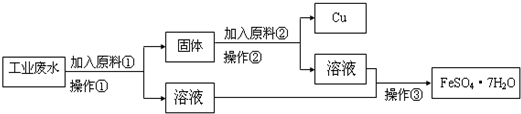
��ش�
��1����Ӧ�ٵ����ӷ���ʽΪ
��2�����������õ��IJ���������
a���ձ�b��©��c��������d���ƾ���
��3��ԭ�Ϣڵ�����Ϊ
��4��������������Һ�μӵ�����������Һ�У��۲쵽������Ϊ

��ش�
��1����Ӧ�ٵ����ӷ���ʽΪ
Cu2++Fe�TCu+Fe2+
Cu2++Fe�TCu+Fe2+
����2�����������õ��IJ���������
abc
abc
������ţ���a���ձ�b��©��c��������d���ƾ���
��3��ԭ�Ϣڵ�����Ϊ
ϡ����
ϡ����
����4��������������Һ�μӵ�����������Һ�У��۲쵽������Ϊ
�����ɰ�ɫ��������Ѹ�ٱ�ɻ���ɫ���ձ�ɺ��ɫ
�����ɰ�ɫ��������Ѹ�ٱ�ɻ���ɫ���ձ�ɺ��ɫ
�йط�Ӧ�Ļ�ѧ����ʽΪFe2++2OH-�TFe��OH��2��4Fe��OH��2+O2+2H2O�T4Fe��OH��3
Fe2++2OH-�TFe��OH��2��4Fe��OH��2+O2+2H2O�T4Fe��OH��3
����������1�����ݸ���Ҫ����ͭ�Ҳ������µ������ж�ԭ�Ϣ٣���д����Ӧ�����ӷ���ʽ��
��2�����ݻ�����״̬ȷ��ʵ�����������ʵ�����ȷ��ʵ��������
��3�����жϲ����ٺ�Ĺ���ɷ֣��ٸ�������ͼ֪�������ٺ����Һ�е�����������ں����Һ�е�������ͬ�ж�ԭ�Ϣڣ�
��4�������������������������ӷ�Ӧ�����������������������Ȼ��д����Ӧ�����ӷ���ʽ��
��2�����ݻ�����״̬ȷ��ʵ�����������ʵ�����ȷ��ʵ��������
��3�����жϲ����ٺ�Ĺ���ɷ֣��ٸ�������ͼ֪�������ٺ����Һ�е�����������ں����Һ�е�������ͬ�ж�ԭ�Ϣڣ�
��4�������������������������ӷ�Ӧ�����������������������Ȼ��д����Ӧ�����ӷ���ʽ��
����⣺��1����������֪���������ܺ�ͭ���ӷ�Ӧ�Ҳ������µ��������ӣ���������Ϊ��������ͭ���ӷ�Ӧ�����ӷ���ʽΪ��Cu2++Fe�TCu+Fe2+��
�ʴ�Ϊ��Cu2++Fe�TCu+Fe2+��
��2�������ڵķ����ǹ��塢Һ����룬����Ӧ�ù��˵ķ��������õ��IJ��������У�a���ձ�b��©��c����������
�ʴ�Ϊ��abc��
��3���������м��������������ʹͭ������ȫ��Ӧ���������ù���ijɷ�������ͭ����������ͭ�һ���ͭ�����Լ���ij����������Ӧ����ͭ����Ӧ����������Ӧ��������������������Һ�����ʵijɷ�һ�������������Ϊϡ���ᣬ
�ʴ�Ϊ��ϡ���
��4��������������Һ�μӵ�����������Һ�У��۲쵽������Ϊ�������ɰ�ɫ������������������Ѹ�ٱ�ɻ���ɫ���ձ�ɺ��ɫ��������������Ӧ�����ӷ���ʽΪ��Fe2++2OH-�TFe��OH��2��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ�������ɰ�ɫ��������Ѹ�ٱ�ɻ���ɫ���ձ�ɺ��ɫ��Fe2++2OH-�TFe��OH��2��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ��Cu2++Fe�TCu+Fe2+��
��2�������ڵķ����ǹ��塢Һ����룬����Ӧ�ù��˵ķ��������õ��IJ��������У�a���ձ�b��©��c����������
�ʴ�Ϊ��abc��
��3���������м��������������ʹͭ������ȫ��Ӧ���������ù���ijɷ�������ͭ����������ͭ�һ���ͭ�����Լ���ij����������Ӧ����ͭ����Ӧ����������Ӧ��������������������Һ�����ʵijɷ�һ�������������Ϊϡ���ᣬ
�ʴ�Ϊ��ϡ���
��4��������������Һ�μӵ�����������Һ�У��۲쵽������Ϊ�������ɰ�ɫ������������������Ѹ�ٱ�ɻ���ɫ���ձ�ɺ��ɫ��������������Ӧ�����ӷ���ʽΪ��Fe2++2OH-�TFe��OH��2��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ�������ɰ�ɫ��������Ѹ�ٱ�ɻ���ɫ���ձ�ɺ��ɫ��Fe2++2OH-�TFe��OH��2��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
���������⿼���˽���ͭ�Ļ��շ��������������������ӵ�ת����֪ʶ����Ŀ�Ѷ��еȣ������ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ