��Ŀ����
��֪A��B��C��D����Ԫ�����ڱ���ǰ36���е�Ԫ�أ���ԭ���������ε�����������ؽṹ��������Ϣ���±���
Ԫ�� �ṹ��������Ϣ
A ԭ�Ӻ�����һ��δ�ɶԵ��ӣ����⻯����ˮ���Ӽ����γ����
B ԭ�Ӻ���M���������N���������4��
C ��ʹ����Ϊ�㷺�ĺϽ����Ҫ�ɷ�
D ԭ�Ӹ��ڲ���Ӿ��ѱ��ͣ�����������Ϊ1
�������Ϣ�ش��й����⣺
��1��CԪ�������ڱ��е�λ��Ϊ____ ��DԪ��ԭ�ӵ���Χ�����Ų�ʽΪ
��2���������ʾʽд��A���⻯��ˮ��Һ�д��ڵ��������____ ��
��3��A�������γ�ԭ�Ӹ�����Ϊ2:1����ԭ�ӷ��ӣ��������Ļ��ϼ�Ϊ____ ����ԭ���ӻ����������з��ӵ�����ԭ���ӻ�������ͬ����____
a:CO2 b:SO2 c:NH3 d;CH4
��4��A��B���γ����ӻ�����侧���ṹ����ͼ����ʾ������B���ӵĸ���Ϊ____ ����B��������ҵȾ��A���ӵĸ���Ϊ ��
��5��1183 K����C����ľ�����ͼ����ͼ1����1183 K������ת��Ϊͼ2�������־��������ڽ���Cԭ�Ӽ������ͬ����ͼ1��ͼ2��ʾ���־���ԭ�ӵĿռ�������֮��Ϊ___ _�����ø��ű�ʾ����

��1���������ڢ��壨1�֣� 3d104s1��2�֣�
��2��F��H��F��F��H��O��O��H��F��O��H��O��4�ֻ�������ȷ��ʾ��
��3��+2��1�֣�cd��2�֣�
��4��4��1�֣� 8��1�֣�
��5��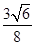 ��0.92��1��68%��74%�Ⱥ����𰸾����֣�3�֣�
��0.92��1��68%��74%�Ⱥ����𰸾����֣�3�֣�
��������
���������ԭ�Ӻ�����һ��δ�ɶԵ��ӣ����⻯����ˮ���Ӽ����γ���������A��FԪ�أ�ԭ�Ӻ���M���������N���������4������˵��M��20��Ԫ��Ca��C��ʹ����Ϊ�㷺�ĺϽ����Ҫ�ɷ֣����C����Ԫ�أ�ԭ�Ӹ��ڲ���Ӿ��ѱ��ͣ�����������Ϊ1����D��29��Ԫ��Cu��
��1����Ԫ�������ڱ��е�λ��Ϊ�������ڢ��壻ͭԪ��ԭ�ӵ���Χ�����Ų�ʽΪ3d104s1��
��2��F��O���ǻ��õķǽ��������γ����������A���⻯��ˮ��Һ�д��ڵ��������ΪF��H��F��F��H��O��O��H��F��O��H��O��
��3��F�ķǽ�����ǿ����Ԫ�أ��ڻ�������Fֻ�У�1�ۣ��������A�������γ�ԭ�Ӹ�����Ϊ2:1����ԭ�ӷ��ӣ����������Ļ��ϼ�Ϊ��2�ۡ��ڸ÷�������Ԫ�غ��еŶԵ��Ӷ�������6��2��1����2��2����� �÷�����V�νṹ����Ԫ����sp3�ӻ���CO2��SO2��NH3��CH4����������ԭ�ӵ��ӻ�������ͷֱ���sp��sp2��sp3��sp3�����Դ�ѡcd��
��4��A��B�γ����ӻ�������CaF2������ݾ����ṹ��֪��������B���ӵĸ���Ϊ��8��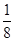 ��6��
��6��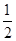 ��4������B��������ҵȾ��A���ӵĸ���Ϊ8����
��4������B��������ҵȾ��A���ӵĸ���Ϊ8����
��5������ԭ�Ӱ뾶��r��������߳�ͼ1��a��ͼ2��b�������ͼ1��֪a2��2a2��(4r)2�����a��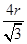 ������ͼ1����ԭ�ӵĿռ���������
������ͼ1����ԭ�ӵĿռ���������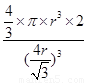 ������ͼ2��֪b2��b2��(4r)2�����b��
������ͼ2��֪b2��b2��(4r)2�����b��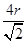 ������ͼ2����ԭ�ӵĿռ���������
������ͼ2����ԭ�ӵĿռ���������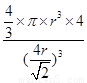 ��������ԭ�ӵĿռ�������֮��Ϊ
��������ԭ�ӵĿռ�������֮��Ϊ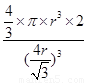 :
: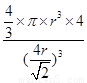 ��
��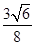 ��
��
���㣺����Ԫ���ƶϣ���������Ų�����������ϼۣ��ӻ�������ͣ�����ṹ���й��жϺͼ����

 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�



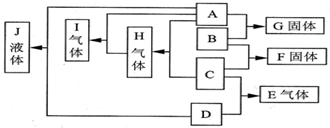

 ��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��
��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��


