��Ŀ����
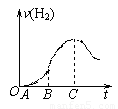
ij��ѧ�о���С����������ϡ����ķ�Ӧ��������ʵ��ʱ���֣�������ڿ����з���һ��ʱ���5.0g��Ƭ����Բ����ƿ�У�Ȼ��ͨ����Һ©������500mL 0.5mol/L������Һ����ַ�Ӧ��С��ͬѧ���ݼ�¼���ݵõ��˲��������������뷴Ӧʱ��Ĺ�ϵͼ����ͼ��ʾ�������������С��ͬѧ�ش��������⣺
��1������OA�β�����������ԭ����______���йص����ӷ�Ӧ����ʽΪ______��
��2������BC�β�������������ͻȻ�ӿ����Ҫԭ����______��
��3����C�Ժ����������������С����Ҫԭ����______��
��4���������Ϸ�����С��ͬѧ���ռ������������Ϊ����״���£�______��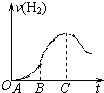
��1������OA�β�����������ԭ����______���йص����ӷ�Ӧ����ʽΪ______��
��2������BC�β�������������ͻȻ�ӿ����Ҫԭ����______��
��3����C�Ժ����������������С����Ҫԭ����______��
��4���������Ϸ�����С��ͬѧ���ռ������������Ϊ����״���£�______��

��1�����ǻ����Խ�ǿ�Ľ�������Ѹ�ٺͿ����е�������Ӧ��������������������ϡ���ᷴӦ�����Ȼ�����ˮ���������ӷ���ʽΪAl2O3+6H+=2Al3++3H2O��
�ʴ�Ϊ��������������Ƭ�����Al2O3��Ӧ�� Al2O3+6H+=2Al3++3H2O��
��2����������ķ�Ӧ�Ƿ��ȷ�Ӧ�����Ը÷�Ӧ���ȣ�ʹ��Һ���¶����ߣ������¶ȣ���ѧ��Ӧ���ʼӿ죮
�ʴ�Ϊ�����ڷ�Ӧ�ų�������ʹ��Һ�¶����߶�ʹ��Ӧ���ʼӿ��ˣ�
��3�����ŷ�Ӧ�Ľ��У���Һ�е�������Ũ�����ͣ����Է�Ӧ������С��
�ʴ�Ϊ�����ŷ�Ӧ�Ľ��У�������Һ��Ũ�����½���
��4����ϡ������ȫ��Ӧ��Ҫ������xg��
2Al+3H2SO4=Al2��SO4��3+3H2��
54g 3mol
xg 0.5L��0.5mol/L
����x=4.5��5���ʽ�����������Ӧ��ϡ����Ϊ���������ɵ����������
���������������Ϊy��
2Al+3H2SO4=Al2��SO4��3+3H2��
3mol 67.2L
0.5L��0.5mol/L y
y=5.6L
�ʴ�Ϊ��5.6 L��
�ʴ�Ϊ��������������Ƭ�����Al2O3��Ӧ�� Al2O3+6H+=2Al3++3H2O��
��2����������ķ�Ӧ�Ƿ��ȷ�Ӧ�����Ը÷�Ӧ���ȣ�ʹ��Һ���¶����ߣ������¶ȣ���ѧ��Ӧ���ʼӿ죮
�ʴ�Ϊ�����ڷ�Ӧ�ų�������ʹ��Һ�¶����߶�ʹ��Ӧ���ʼӿ��ˣ�
��3�����ŷ�Ӧ�Ľ��У���Һ�е�������Ũ�����ͣ����Է�Ӧ������С��
�ʴ�Ϊ�����ŷ�Ӧ�Ľ��У�������Һ��Ũ�����½���
��4����ϡ������ȫ��Ӧ��Ҫ������xg��
2Al+3H2SO4=Al2��SO4��3+3H2��
54g 3mol
xg 0.5L��0.5mol/L
����x=4.5��5���ʽ�����������Ӧ��ϡ����Ϊ���������ɵ����������
���������������Ϊy��
2Al+3H2SO4=Al2��SO4��3+3H2��
3mol 67.2L
0.5L��0.5mol/L y
y=5.6L
�ʴ�Ϊ��5.6 L��

��ϰ��ϵ�д�
�����Ŀ
 ij��ѧ�о���С����������ϡ����ķ�Ӧ��������ʵ��ʱ���֣�������ڿ����з���һ��ʱ���5.0g��Ƭ����Բ����ƿ�У�Ȼ��ͨ����Һ©������500mL 0.5mol/L������Һ����ַ�Ӧ��С��ͬѧ���ݼ�¼���ݵõ��˲��������������뷴Ӧʱ��Ĺ�ϵͼ����ͼ��ʾ�������������С��ͬѧ�ش��������⣺
ij��ѧ�о���С����������ϡ����ķ�Ӧ��������ʵ��ʱ���֣�������ڿ����з���һ��ʱ���5.0g��Ƭ����Բ����ƿ�У�Ȼ��ͨ����Һ©������500mL 0.5mol/L������Һ����ַ�Ӧ��С��ͬѧ���ݼ�¼���ݵõ��˲��������������뷴Ӧʱ��Ĺ�ϵͼ����ͼ��ʾ�������������С��ͬѧ�ش��������⣺