��Ŀ����
T ��ʱ,�мס��������ܱ�����,�����������Ϊ1 L,�����������Ϊ2 L,�ֱ���ס����������м���6 mol A��3 mol B ,���������·�Ӧ��3A��g��+bB��g����1���������з�Ӧ��ƽ������v��B��=__________,��ѧ����ʽ�м���ϵ��b��__________��
��2���������з�Ӧ�ﵽƽ������Ҫ��ʱ��t__________������ڡ���С�ڡ����ڡ���4 min��ԭ����_______________________________��
��3��T ��ʱ������һ���������������ͬ�ı������У�Ϊ��ʹ�ﵽƽ��ʱB��Ũ����ȻΪ0.8 mol��L-1����ʼʱ���������м���C��D�����ʵ����ֱ�Ϊ3 mol ��2 mol���������A��B�����ʵ����ֱ��ǣ�______________________��
��4����Ҫʹ�ס�����������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��__________��
A.�����¶Ȳ��䣬����������������2 L
B.��������������䣬ʹ�����������¶�
C.��������ѹǿ���¶ȶ����䣬����м���һ������A����
D.��������ѹǿ�����䣬����м���һ������B����
˼·��������1����Ӧǰ����������A��Ũ��Ϊ6 mol��L-1��4 min�������ڵķ�Ӧ�ﵽ��ѧƽ���A��Ũ�ȼ�����3.6 mol��L-1��B��Ũ�ȼ�����1.2 mol��L-1�����ݷ�Ӧ���ʵļ��㹫ʽ��ϻ�ѧ��Ӧ����ʽ�и����ʵĻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������v��B��=0.3 mol��L-1��min-1��b��1����2����������������ڼģ�Ũ��С����ƽ���ʱ�䳤����3�����ݵ�Чƽ����ɣ��ں��º����£�ת��Ϊͬһ��Ӧ��������ʵ����ʵ�����ԭ����Ӧ��ȼ��ɡ�3 mol C��2 mol D��ȫת��������3 mol A��1 mol B������3 mol A��2 mol B����4��������������ԭ������ϵ�Чƽ����ɣ���֪A��B��C���С�
�𰸣���1��0.3 mol��L-1��min-1 1
��2������ ��������������ڼ��������������Ӧ��Ũ�ȼ�С����Ӧ���ʼ������ﵽƽ������Ҫ��ʱ���Ҫ��
��3��3 mol��2 mol
��4��ABC

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� 3C��g����2D��g����
3C��g����2D��g����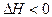 ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺ 3C��g����2D��g����
3C��g����2D��g���� ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺