��Ŀ����
�Ժ�������ʯΪԭ����������Ĺ�������ͼ���£�

��ش��������⣺
��1����ȼ�պ�������ʯ�Ļ�ѧ����ʽ����������4______+11O2 2Fe2O3+8SO2��
2Fe2O3+8SO2��
������պ�������ʯWg����Ӧ��ȫ����ȴ�����£��Ƶù������ʵ�������mg������SO2ΪVL����������ʯ����Ԫ�ص����������ǣ������ʯ�е��������Ȳ��ֽ⣬Ҳ������SO2��Ӧ��______��
A.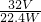 ����B.
����B.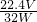 ����C.
����C.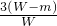 �� D.
�� D.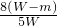
��2���Ӵ����з�����Ӧ�Ļ�ѧ����ʽ��______��
ij�¶��£�����Ӵ�����SO2��O2�����ʵ�����Ϊ1��1����Ӧ�ﵽƽ��ʱ��ѹǿ���� ����SO2��ת����Ϊ______��
����SO2��ת����Ϊ______��
��3�����ݹ�������ͼ�ж�����˵����ȷ���ǣ�ѡ�������ĸ��______��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
��4����������������SO3�����X������______��д���ƣ���
��֪ÿ240g SO3������H2O���Ϸų�390.9kJ���������÷�Ӧ���Ȼ�ѧ������______��
��5���ų���β��SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ��______��
�⣺��1������ʯ�ֽл�������Ҫ����FeS2������FeS2��������Ӧ��ȡ�����������壻���ݻ�ѧ����ʽ����ʯ�е���Ԫ��ת��Ϊ������������Ԫ���غ�ɼ�����Ԫ��������ʯ�еĺ��������ù���仯���㣻
4FeS2+11O2 2Fe2O3+8SO2 ������������
2Fe2O3+8SO2 ������������
8��64��m=512-352=160
m��SO2�� �� W-m��g
m��SO2��=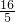 ��W-m��g��
��W-m��g��
n��SO2��=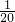 ��W-m��mol��
��W-m��mol��
������ʯ����Ԫ�ص���������= ��100%=
��100%=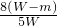 ��
��
�ʴ�Ϊ����1��FeS2��D
��2���Ӵ�����Ҫ�����ô����Ѷ��������ڴ�����������Ϊ�����������Ի�ѧ����ʽΪ2SO2+O2 3SO3��
3SO3��
ij�¶��£�����Ӵ�����SO2��O2�����ʵ�����Ϊ1��1����Ӧ�ﵽƽ��ʱ��ѹǿ���� ����SO2��ת���ʸ���ƽ����㣬���������Ӧ�����ʵ���Ϊx
����SO2��ת���ʸ���ƽ����㣬���������Ӧ�����ʵ���Ϊx
2SO2+O2 2SO3
2SO3
��ʼ�� 1 1 0
�仯�� x 0.5x x
ƽ����1-x 1-0.5x x
��Ӧ�ﵽƽ��ʱ��ѹǿ���� ������1-x ��+�� 1-0.5x��+x=2��1-
������1-x ��+�� 1-0.5x��+x=2��1- ����x=0.8��
����x=0.8��
���Զ�������ת����Ϊ80%��
�ʴ�Ϊ��2SO2+O2 3SO3��80%��
3SO3��80%��
��3��a��Ϊʹ��������ȼ�գ��轫����飬Ŀ�������ӽӴ������߷�Ӧ���ʣ�����ȷ��
b���������������SO2��ת���ʣ�����������������߶��������ת���ʣ�����ȷ��
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת���ʣ���������߷�Ӧ���ʣ������ı�ƽ�⣬ת���ʲ��䣬�ʴ���
d������¯�ų��Ŀ����ɹ����������������ɵ������������ڹ�ҵ����������ȷ��
��ѡabd��
��4����ˮ���������������γ�����������������������գ���ҵ����98.3%��Ũ����������������
ÿ240g SO3������H2O���Ϸų�390.9kJ����������3molSO3��ˮ���Ϸ���390.9kJ��1molSO3��H2O���Ϸų�130.3kJ��������д�Ȼ�ѧ����ʽ��Ҫ��д����SO3��g��+H2O��l���TH2SO4��l������H=-130.3kJ/mol��
�ʴ�Ϊ��Ũ��� SO3��g��+H2O��l���TH2SO4��l������H=-130.3kJ/mol��
��5���嵥����ǿ�����������Ѷ�����������Ϊ���ᣬ��������ԭΪ�����ӣ����������ԭ��Ӧ����������д�����ӷ���ʽ��ΪSO2+Br2+2H2O�T4H++2Br-+SO42-��
�ʴ�Ϊ��SO2+Br2+2H2O�T4H++2Br-+SO42-
�������������������Ϲ�ҵ������IJ����ԭ�����з����жϺͼ��㣻
��1����Ҫ������������ijɷֺͻ�ѧʽ����д��ͬʱ���ݻ�ѧ����ʽ�����������������Ԫ�صĺ�����
��2���Ӵ����з�����Ӧ�ǰѶ��������ڴ����ı�����д�����������������
��3�������ʺͻ�ѧƽ��Ƕȷ���Ӱ�췴Ӧ���ʺ�ƽ������أ�
��4����ҵ�������������������������γ�����������ˮ���գ�������Ũ�����Ϸ�Ӧ������ȷ��д�Ȼ�ѧ����ʽ��
��5�����϶�������Ļ�ѧ���ʣ�����ǿ���������ֻ�ԭ�ԣ�����ǿ��ԭ�����������ԣ�
���������⿼���˹�ҵ �������ԭ����ԭ�ϵijɷֺͺ������㡢���������е���Ҫ�������⣬�ص㿼���˻�ѧƽ���Ӱ�����ء�ƽ������֪ʶ��
4FeS2+11O2
 2Fe2O3+8SO2 ������������
2Fe2O3+8SO2 ������������8��64��m=512-352=160
m��SO2�� �� W-m��g
m��SO2��=
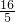 ��W-m��g��
��W-m��g��n��SO2��=
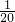 ��W-m��mol��
��W-m��mol��������ʯ����Ԫ�ص���������=
 ��100%=
��100%=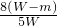 ��
���ʴ�Ϊ����1��FeS2��D
��2���Ӵ�����Ҫ�����ô����Ѷ��������ڴ�����������Ϊ�����������Ի�ѧ����ʽΪ2SO2+O2
 3SO3��
3SO3��ij�¶��£�����Ӵ�����SO2��O2�����ʵ�����Ϊ1��1����Ӧ�ﵽƽ��ʱ��ѹǿ����
 ����SO2��ת���ʸ���ƽ����㣬���������Ӧ�����ʵ���Ϊx
����SO2��ת���ʸ���ƽ����㣬���������Ӧ�����ʵ���Ϊx2SO2+O2
 2SO3
2SO3 ��ʼ�� 1 1 0
�仯�� x 0.5x x
ƽ����1-x 1-0.5x x
��Ӧ�ﵽƽ��ʱ��ѹǿ����
 ������1-x ��+�� 1-0.5x��+x=2��1-
������1-x ��+�� 1-0.5x��+x=2��1- ����x=0.8��
����x=0.8�����Զ�������ת����Ϊ80%��
�ʴ�Ϊ��2SO2+O2
 3SO3��80%��
3SO3��80%����3��a��Ϊʹ��������ȼ�գ��轫����飬Ŀ�������ӽӴ������߷�Ӧ���ʣ�����ȷ��
b���������������SO2��ת���ʣ�����������������߶��������ת���ʣ�����ȷ��
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת���ʣ���������߷�Ӧ���ʣ������ı�ƽ�⣬ת���ʲ��䣬�ʴ���
d������¯�ų��Ŀ����ɹ����������������ɵ������������ڹ�ҵ����������ȷ��
��ѡabd��
��4����ˮ���������������γ�����������������������գ���ҵ����98.3%��Ũ����������������
ÿ240g SO3������H2O���Ϸų�390.9kJ����������3molSO3��ˮ���Ϸ���390.9kJ��1molSO3��H2O���Ϸų�130.3kJ��������д�Ȼ�ѧ����ʽ��Ҫ��д����SO3��g��+H2O��l���TH2SO4��l������H=-130.3kJ/mol��
�ʴ�Ϊ��Ũ��� SO3��g��+H2O��l���TH2SO4��l������H=-130.3kJ/mol��
��5���嵥����ǿ�����������Ѷ�����������Ϊ���ᣬ��������ԭΪ�����ӣ����������ԭ��Ӧ����������д�����ӷ���ʽ��ΪSO2+Br2+2H2O�T4H++2Br-+SO42-��
�ʴ�Ϊ��SO2+Br2+2H2O�T4H++2Br-+SO42-
�������������������Ϲ�ҵ������IJ����ԭ�����з����жϺͼ��㣻
��1����Ҫ������������ijɷֺͻ�ѧʽ����д��ͬʱ���ݻ�ѧ����ʽ�����������������Ԫ�صĺ�����
��2���Ӵ����з�����Ӧ�ǰѶ��������ڴ����ı�����д�����������������
��3�������ʺͻ�ѧƽ��Ƕȷ���Ӱ�췴Ӧ���ʺ�ƽ������أ�
��4����ҵ�������������������������γ�����������ˮ���գ�������Ũ�����Ϸ�Ӧ������ȷ��д�Ȼ�ѧ����ʽ��
��5�����϶�������Ļ�ѧ���ʣ�����ǿ���������ֻ�ԭ�ԣ�����ǿ��ԭ�����������ԣ�
���������⿼���˹�ҵ �������ԭ����ԭ�ϵijɷֺͺ������㡢���������е���Ҫ�������⣬�ص㿼���˻�ѧƽ���Ӱ�����ء�ƽ������֪ʶ��

��ϰ��ϵ�д�
�����Ŀ


 ��ʵ�ʹ�ҵ�����У������á���ת ����������������һ��ת�����ɵ�SO2�����δת����SO2���ж���ת������������SO2��ת���ʾ�Ϊ95%��������SO2��ת����Ϊ ��
��ʵ�ʹ�ҵ�����У������á���ת ����������������һ��ת�����ɵ�SO2�����δת����SO2���ж���ת������������SO2��ת���ʾ�Ϊ95%��������SO2��ת����Ϊ ��