��Ŀ����
50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

��1����ʵ��װ�ÿ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��_______________��
��2���ձ���������ֽ����������_________________________��
��3��ʵ���и���60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����Ƚϣ����ų�������__________�����ȡ�����ȡ������к���__________�����ȡ�����ȡ�����������__________________________________��
��4����50mL0.50mol/L�Ĵ������������Һ��������ʵ�飬����к��ȵ���ֵ��57.3kJ/mol��Ƚϻ�____________�����ƫ����ƫС������Ӱ�족����
��5����������һ��������ʵ�飬��ⶨ______���¶ȡ�
��6�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ����ƫС������Ӱ�족)
��1�����β�������� ��2������ʵ�������������ʧ
��3������� ��� ��Ϊ�к�����ָ��ϡ��Һ�У��������кͷ�Ӧ����1molH2O���ų�������������������� ��ƫС ��5��3 ��6��ƫС
�������������к��ȵIJⶨ����������
��1����ʵ������У���ʹ��Һ��Ͼ��ȣ���Ҫ���裬��˺�ȱ�ٵ������ǻ��β����������
��2����ʵ������У���Ҫ�����ܵļ�����������ʧ�������ձ���������ֽ���������Ǽ���ʵ�������������ʧ��
��3�������к�����ָ��ϡ��Һ�У��������кͷ�Ӧ����1molH2O���ų�������������������ء����Ըı��������������Ӧ�зų����������Ըı䣬���к����Dz���ġ�
��4��������������ʣ����ڵ���ƽ�⡣�����������ȵģ����Բⶨ����ֵ��ƫС��
��5��Ϊ�˼�Сʵ����Ӧ�����ٲⶨ3���¶ȣ�Ȼ������ƽ��ֵ��
��6�����ձ����粻��Ӳֽ�壬����������ʧ����˵õ��к�����ֵƫС��

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺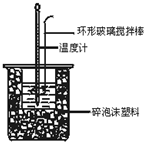 �к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵������ȷ���ǣ�������
�к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵������ȷ���ǣ������� ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺ 50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺
50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺