��Ŀ����
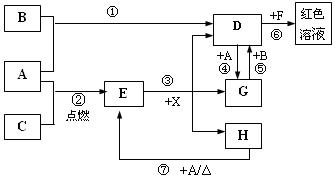
��11�֣�A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ�������AΪ���塢BΪҺ�塢CΪ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����
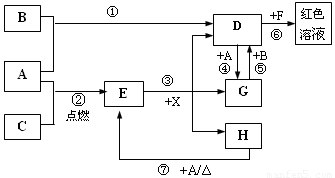
��1��д���������ʵĻ�ѧʽ�� D___________�� X____________��
��2���ڷ�Ӧ�١����У�������������ԭ��Ӧ����______�����ţ���
��3����Ӧ�����ӷ���ʽΪ��_____________________________________________________
��4����G��Һ�м���NaOH��Һ�۲쵽��������
��5����Ӧ�ߵĻ�ѧ����ʽΪ______________________________________________________��
�÷�Ӧ��ÿ����0��3 mol��A����ת�Ƶ���_______________mol��
��6��д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽΪ________________________________��
��1�� FeBr3�� HBr ��2���ۢ�
��3��Fe3+ +3SCN-=Fe��SCN��3��
��4�����ɵij����ɰ�ɫ��Ϊ����ɫ���ձ�Ϊ���ɫ��
��5��3Fe+4H2O��g�� Fe3O4+4H2
0��8
Fe3O4+4H2
0��8
��6��Fe3++3HCO3-= Fe��OH��3��+3CO2��
����������

 ͬ����ϰǿ����չϵ�д�
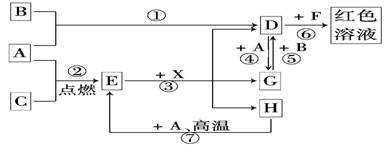
ͬ����ϰǿ����չϵ�д�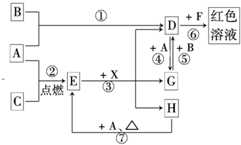 ��2012?ʯ��ɽ��һģ��A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬CΪ���壮D��E��F��G��H��X��Y��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�壮����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ����
��2012?ʯ��ɽ��һģ��A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬CΪ���壮D��E��F��G��H��X��Y��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�壮����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ����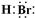
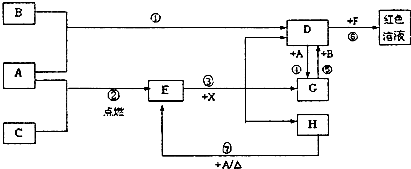
 ������ͨ�������AΪ
������ͨ�������AΪ ���塢BΪҺ�塢CΪ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����
���塢BΪҺ�塢CΪ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����