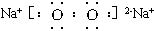��Ŀ����
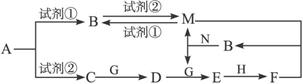
�������ʾ�Ϊ��ѧ��ѧ���������ʣ���ת����ϵ��ͼ��ʾ����Ӧ���������ֲ���δȫ���������֪AΪ���Σ��Լ��ٺ��Լ���Ϊ��ѧ��ѧʵ���ҳ��õ��Լ�������B��C��D��E��G������Ϊ���壬H������ΪҺ�塣
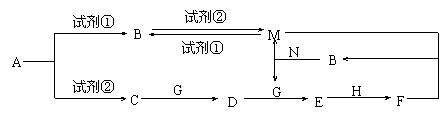 ��1��A�Ļ�ѧʽΪ ��
��1��A�Ļ�ѧʽΪ ��
��2��N�ĵ���ʽΪ ��������ѧ��Ϊ ��
��3��A��C�����ӷ���ʽΪ ��
��4��C��D�Ļ�ѧ����ʽΪ ��
��5����֪��״������33.6L��C��G��ȫ��Ӧ����D����ת�Ƶĵ�����Ϊ NA��
��1��(NH4)2CO3
��2��Na2O2�ĵ���ʽ�� ���Ӽ��ͷǼ��Լ�
��3��![]()
��4��![]()
��5��7.5

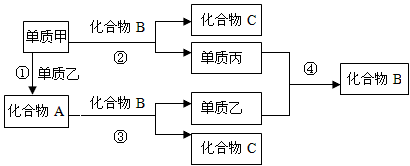
��ϰ��ϵ�д�
�����Ŀ