��Ŀ����
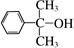
ij�ܻ�����DEHP���Ľṹ��ʽΪ��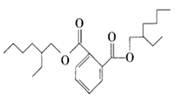
��ϳ�·�����£�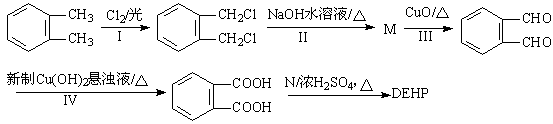
��1��DEHP�ķ���ʽ ��
��2����ӦI������ ����ӦIV�IJ����к��������ŵ����� ��
��3��M��N�Ľṹ��ʽ�ֱ�ΪM ��N ��
��4����ӦIV�Ļ�ѧ����ʽ ��
��5��һ�������£�1mol ��������H2��Ӧ����������H2 mol��
��������H2��Ӧ����������H2 mol��
��6������ʽΪC8H6Cl4��һ��ͬ���칹�����ͨ�����ƣ�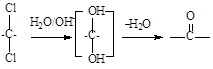 �ķ�Ӧ�õ�
�ķ�Ӧ�õ� ����д����ͬ���칹��Ľṹ��ʽ�� ��
����д����ͬ���칹��Ľṹ��ʽ�� ��
��16�֣�
��1��C14H18O4��2�֣�
��2��ȡ����Ӧ��1�֣� �Ȼ���1�֣�
��3��  ��2�֣� CH3CH2CH2OH��2�֣�
��2�֣� CH3CH2CH2OH��2�֣�
��4��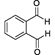 + 4 Cu(OH)2
+ 4 Cu(OH)2 
 + 2 Cu2O��+4H2O��3�֣�
+ 2 Cu2O��+4H2O��3�֣�
��5�� 5 ��2�֣�
��6��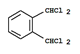 ��3�֣�
��3�֣�
�������������
��1���ɼ���ʽת��Ϊ����ʽ����д��Ҫ�㣺���㡢ת�۵�̼ԭ�Ӳ�Ҫ��������̼���⡢��ԭ�ӵ���Ŀһ�����壬�ã�C14H18O4��
��2����Ӧ���͵��жϣ�I��IIΪȡ����Ӧ��III��IVΪ������Ӧ��IV�IJ������ڱ������ᣬ���������Ȼ���
��3���ṹ�ƶϣ�MΪ�ڱ����״���N��1-������д����ṹ��ʽ���ɣ�
��4��д���ڱ�����ȩ������Cu(OH)2����Ϊ�ڱ�������Ļ�ѧ����ʽ��ע����ƽ�ͷ�Ӧ������ҪŪ����
��5��1mol�����������3molH2�ӳɣ�1molȩ������1molH2���ϼƹ�����5molH2��
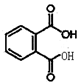
��6����������Ϣ������ʽΪ��C8H6Cl4��ˮ��õ��ڱ�����ȩ�ĽṹΪ��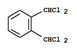 ��
��
���㣺�����л���ѧ�ۺ������������漰�����У��ɼ���ʽд����ʽ���жϷ�Ӧ���͡�ʶ������š��ƶϽṹ��ʽ����дȩ������������ͭ�����Ļ�ѧ����ʽ����������������������⡢��Ϣ����������������ͬ���칹�����д��

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�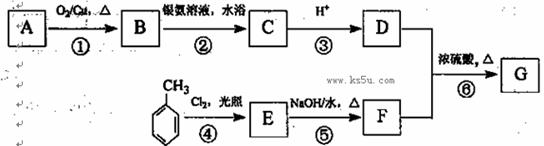
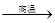 CO��+H2�������ᱽ����(F)���������Ϻ�ҩ�����Ҫԭ�ϡ���ͼ����úΪԭ�Ϻϳɼ��ᱽ������·��ͼ�����ַ�Ӧ����������������ȥ������D�ķ���ʽΪC9H10O�����ܷ���������Ӧ��
CO��+H2�������ᱽ����(F)���������Ϻ�ҩ�����Ҫԭ�ϡ���ͼ����úΪԭ�Ϻϳɼ��ᱽ������·��ͼ�����ַ�Ӧ����������������ȥ������D�ķ���ʽΪC9H10O�����ܷ���������Ӧ��
 ��1��������D�й����ŵ�����Ϊ �� ��������
��1��������D�й����ŵ�����Ϊ �� ��������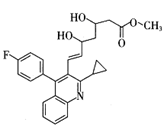 ����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� ��
����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� �� ���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����
���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д���� Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ�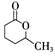 �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�CH3CH2OH
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�CH3CH2OH CH2��CH2
CH2��CH2 CH3CH3
CH3CH3
 ��������
��������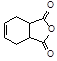 �Ǻϳɿ�����ҩ������Τ���м��壬����ƺ���������
�Ǻϳɿ�����ҩ������Τ���м��壬����ƺ��������� ��
�� Ϊԭ�Ϻϳɸû�����úϳ�·������ͼ��ʾ����ע����Ӧ���������ϳ�·������ͼʾ�����£�
Ϊԭ�Ϻϳɸû�����úϳ�·������ͼ��ʾ����ע����Ӧ���������ϳ�·������ͼʾ�����£� 

 ����λ�칹���������ˮ�����㶹�صĽṹ��ʽ ��
����λ�칹���������ˮ�����㶹�صĽṹ��ʽ �� )��ԭ�ϣ�д������A����ȫת��Ϊ�������ķ�����
)��ԭ�ϣ�д������A����ȫת��Ϊ�������ķ�����
 Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��______________��
Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��______________��