��Ŀ����
(1)
�� ��ʹ�����ʴ�ˮ��Һ���������ֲ��ı�������Ҫ���ʣ�Ӧ����________(����ĸ)��
a. �������֡� b. ������������Һ�� c. ����ͭ��Һ
�� ���𱽺ͼױ��ķ������Լ���________(����ĸ)��
a. Ũ��ˮ �� b. �ữ��KMnO4��Һ �� c. �ڿ����е�ȼ
�� ���л������У����³�ѹ��Һ̬����________(����ĸ)��
a. �״� �� b. ��Ȳ �� c. ��ϩ
(2) �Ʊ�������������Ҫ�����������ʣ�
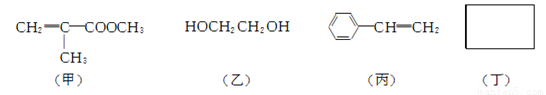
�� ���в�����ԭ�ӵĹ�������________(������)��
�� ����ͨ������ת�����Եõ���(����A��D��Ϊ�л���)��
 A�Ļ�ѧʽ��________��C��D�ķ�Ӧ������________��
A�Ļ�ѧʽ��________��C��D�ķ�Ӧ������________��
�� �������Ӿ۷�Ӧ��������Ϊ________(д�ṹ��ʽ)��
�� �����ﶡ����̼���⡢������Ԫ�أ���Է�������Ϊ110������FeCl3��Һ��������ɫ���Ҷ������������ϵ�һ��ȡ����ֻ��һ�֡��Ľṹ��ʽΪ________��
(3) A��F��ת����ϵ����(��֪A��������)��

�� �л���A����Է�������Ϊ90��9 g A������Na��Ӧ������2.24 L H2(��״��)����A�ķ���ʽΪ________��
�� A�������ɸ߷��ӻ�����Ľṹ��ʽΪ________��
�� ��E����F�ķ�Ӧ������________��
�� ��D����E�Ļ�ѧ����ʽΪ________��
�� д����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��________��
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�



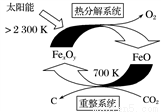
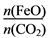 ��6����FexOy�Ļ�ѧʽΪ________��
��6����FexOy�Ļ�ѧʽΪ________��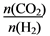 ֵ��С
ֵ��С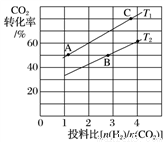
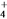 )________(�>������<������)c(HCO
)________(�>������<������)c(HCO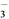 )����ӦNH
)����ӦNH ��HCO
��HCO ��̼ԭ������10)����֪�����������ֻ�ѧ������ͬ����ԭ�ӣ�����Ŀ֮��Ϊ3��2�������һ���DZ���ͬϵ��
��̼ԭ������10)����֪�����������ֻ�ѧ������ͬ����ԭ�ӣ�����Ŀ֮��Ϊ3��2�������һ���DZ���ͬϵ��
 S��+2NO2��+2H2O
S��+2NO2��+2H2O 4NO2��+O2��+2H2O
4NO2��+O2��+2H2O
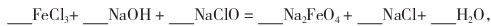
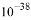 �����¶�������ʵ����������5mol/L l00mL FeCl3��Һ��Ϊʹ���ƹ����в����ֻ���������������Ҫ���� mL 2 mol/L�����ᣨ���Լ��������������
�����¶�������ʵ����������5mol/L l00mL FeCl3��Һ��Ϊʹ���ƹ����в����ֻ���������������Ҫ���� mL 2 mol/L�����ᣨ���Լ��������������