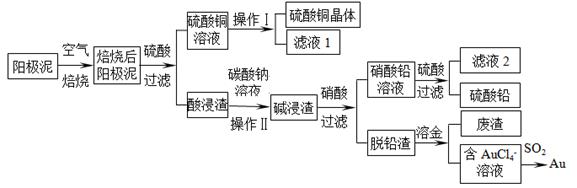
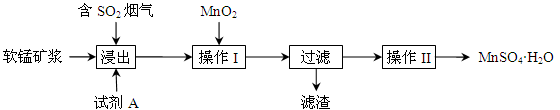
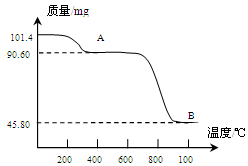
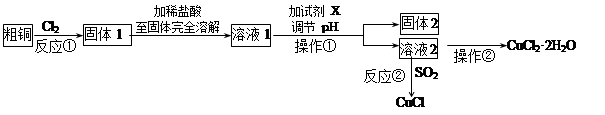��Ŀ����
��������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ�����Ƶ����̣�
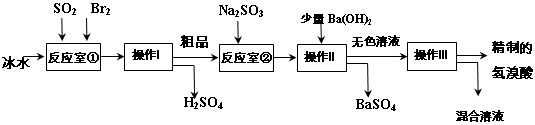
��֪��Br2���ӷ��������ɫ��Һ�壻���������ӷ�����ɫҺ�塣
�����������̻ش��������⣺
��1����Ӧ�Ң��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ�� ��
��3������I������ ���������õ��IJ��������� ��
��4����Ӧ�Ң��м���Na2SO3��Ŀ���� ��
��5����ҵ�������Ƶõ���������е����Ļ�ɫ�����Ǽ�����ͬѧ�����ʵ�����̽����
�ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ ��������֤���ü�������ķ���Ϊ ��

��֪��Br2���ӷ��������ɫ��Һ�壻���������ӷ�����ɫҺ�塣
�����������̻ش��������⣺
��1����Ӧ�Ң��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ�� ��
��3������I������ ���������õ��IJ��������� ��
��4����Ӧ�Ң��м���Na2SO3��Ŀ���� ��
��5����ҵ�������Ƶõ���������е����Ļ�ɫ�����Ǽ�����ͬѧ�����ʵ�����̽����
�ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ ��������֤���ü�������ķ���Ϊ ��
��1��SO2+Br2+2H2O=H2SO4+2HBr ��2����ֹBr2��HBr�ӷ�
��3������©�������������ձ��� ��4����ԭ��Ʒ�е�Br2
��5����KSCN��Һ����������KSCN��Һ���ɫ�����������к���Br2���ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ��������ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��
��3������©�������������ձ��� ��4����ԭ��Ʒ�е�Br2
��5����KSCN��Һ����������KSCN��Һ���ɫ�����������к���Br2���ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ��������ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��
�����������1����Ӧ�Ң���SO2��Br2�ڱ�ˮ�з�����Ӧ�Ļ�ѧ����ʽΪSO2+Br2+2H2O=H2SO4+2HBr����2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ����Ϊ�˷�ֹBr2��HBr�ӷ�����Ⱦ������Ӱ���������3������I�Ƿ��뻥�ܵķе㲻ͬ��Һ�����ʵIJ��������������������õ��IJ���������©�������������ձ�����4����Ӧ�Ң��м���Na2SO3��Ŀ����Ϊ������Ϊ��Ӧ��Br2��������ʵĴ��ȡ���5����ҵ�������Ƶõ���������е����Ļ�ɫ���ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3��������ķ�����ȡ��������Һ�������еμӼ���KSCN��Һ������ҵ��������KSCN��Һ���ɫ����֤������Fe3��������Ͱɺ���Fe3��������ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ����Br2������ķ�����������ǿ�����ԡ��ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ�����Ҳ�������������л��ܼ��е��ܽ�ȴ�����ʡ��ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��2��Br2�����ʡ������ķ��뷽����������Fe3����Br2�ļ��鷽����֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
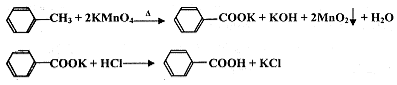
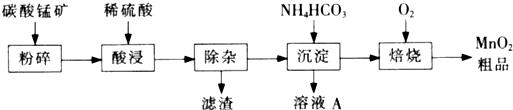 �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���