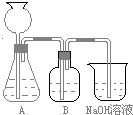
��Ŀ����
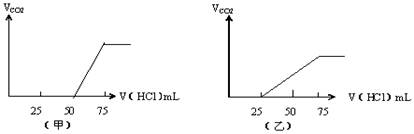
��50mLNaOH��Һ����������ͨ��һ������CO2�����ȡ����Һ10mL����ϡ�͵�100mL�������ϡ�ͺ����Һ����μ���0.1mol/L��HCl��Һ��������CO2�����������״���£����������HCl�����֮��Ĺ� ϵ��ͼ��ʾ���Է�����
��NaOH������CO2������ڼס��� ��������£�������Һ�д��ڵ�������
�ף� �����ʵ���֮���ǣ�
�ң� �����ʵ���֮���ǣ�
��.������������²�����CO2���壨��״����
���Ǽף� mL���ң� mL��
��.ԭNaOH��Һ�����ʵ���Ũ���� ��
���𰸡�
��
�� NaOH��Na2CO3�� ��1�֣� 1:1��1�֣���
NaHCO3��Na2CO3����1�֣� 1:1��1�֣���
�� 56mL����1�֣� 112mL����1�֣�
�� 0.75mol/L����2�֣�

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д� �żӾ���ϵ�д�
�żӾ���ϵ�д�
�����Ŀ