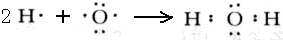��Ŀ����
X��Y��ZΪԭ��������С�������е����ֶ�����Ԫ�ء���֪��X2Z������X2Y2���Ӿ�����ͬ�ĵ�������Y��Zͬ���壬�����γ�ZY2��ZY3���ۻ������ش�
(1)Z������������Ӧˮ����w����Ҫ�Ļ���ԭ�ϣ�W�Ļ�ѧʽΪ___________________��X2Y2�ĵ���ʽΪ_______________________________________________________��
(2)��ҵ������W�Ĺ�����Ҫ��Ϊ�����Σ�����IJ����������Σ�д���ò���Ӧ�Ļ�ѧ����ʽ______________________���жϸ÷�Ӧ���ﻯѧƽ��ı�־��____________________��
A.�����ʵ�Ũ�����
B.�����ʵ�Ũ�Ȳ��ٸı�
C.�����ʵķ�Ӧ�������
D.�ݻ��̶��������У������¶Ȳ��䣬ѹǿ���ٸı�
(3)�����������Ĺ����У�����ȡ�Ĵ�ʩ�У�
���¶ȿ���
�������Ƚ������������ǣ�_________________________________________________________ __________________________________________________________________��
(4)���ڷ�Ӧ��ʼʱͶ��2 mol ZY2��1 mol Y2����Ӧ����ƽ�����ϵѹǿ��С30%����÷�Ӧ��ת����Ϊ_________________________________________________________________��
(1)H2SO4 ![]()
(2)2SO2(g)+O2(g)![]() 2SO3(g) BD
2SO3(g) BD
(3)�÷�Ӧ�Ƿ��ȷ�Ӧ�����������ʿ쵫���ʵͣ����²��ʸ�ȴ��������
(4)90��

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�