��Ŀ����
��һ����ɫ���壬���ܺ���CaCO3��Na2SO4��KNO3��CuSO4��BaCl2���������е�һ�ֻ��֡��ֽ�������ʵ�飺
��1��ȡ���������ĩ�ӵ�����ˮ�У��õ���ɫ�������ϲ�Ϊ��ɫ��Һ��
��2�����������м�������ϡ���ᣬ��ɫ������ȫ��ʧ���������ݲ�����
��3��ȡ�������е���Һ�μ�Ba(NO3)2��Һ���а�ɫ�������ɣ��ټ���ϡ���ᣬ�������ܡ�
��������ʵ�������жϣ��ð�ɫ������һ������__________________��һ��������________�����ܺ���_____________��
�����֤���ܴ��ڵ�����
һ������CaCO3��Na2SO4����2�֣���һ��������CuSO4��BaCl2����2�֣������ܺ���KNO3����1�֣���
ȡ����������ɫ��Ӧ������ɫ�ܲ������������ɫ��֤����KNO3��2�֣�
����������������ݣ�1�����õ���ɫ�������ϲ�Ϊ��ɫ��Һ����˵����Һ�в�����CuSO4�����г�������ΪBaSO4��CaCO3�����ݣ�2���������ɫ������ʧ��ȷ����������BaSO4ӦΪCaCO3������(3)�ж���Һ�к���SO42-����ԭ�����к���Na2SO4������ȷ������KNO3��������ɫ��Ӧ��������ӣ�ȷ��KNO3�Ƿ���ڡ�
���㣺�������ӹ����й����⡣

�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�
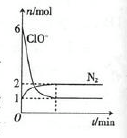
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
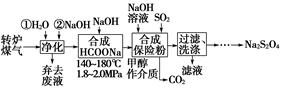
 8Cu��4FeO��2Fe2O3��16SO2
8Cu��4FeO��2Fe2O3��16SO2