��Ŀ����
�״�(CH3OH)��һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϣ���֪��
CH3OH(l)��O2(g)===CO(g)��2H2O(g) ��H1����443.64 kJ/mol
2CO(g)��O2(g)===2CO2(g)����H2����566.0 kJ/mol
(1)��д��CH3OH(l)����������ȫȼ������CO2��H2O(g)���Ȼ�ѧ����ʽ��______________________________________________.
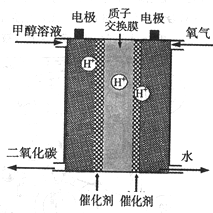
(2)������Ա�½�������һ����ǿ�����������Һ�����ͼ״��ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε磬�ݴ˻ش𣺼״���________����Ӧ��
(3)���õ�ؿ�ʵ�ֵ�����ѧ�ܵ�ת����ijͬѧ�����һ���õ�ⷨ��ȡFe(OH)2��ʵ��װ��(��ͼ)��ͨ�����Һ�в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ������˵���в���ȷ����________(�����)��
A����Դ�е�aһ��Ϊ������bһ��Ϊ����
B��������NaCl��Һ��Ϊ�������Һ
C��A��B���˶������������缫
D�����������ķ�Ӧ��2H����2e��===H2��
CH3OH(l)��O2(g)===CO(g)��2H2O(g) ��H1����443.64 kJ/mol
2CO(g)��O2(g)===2CO2(g)����H2����566.0 kJ/mol
(1)��д��CH3OH(l)����������ȫȼ������CO2��H2O(g)���Ȼ�ѧ����ʽ��______________________________________________.
(2)������Ա�½�������һ����ǿ�����������Һ�����ͼ״��ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε磬�ݴ˻ش𣺼״���________����Ӧ��
(3)���õ�ؿ�ʵ�ֵ�����ѧ�ܵ�ת����ijͬѧ�����һ���õ�ⷨ��ȡFe(OH)2��ʵ��װ��(��ͼ)��ͨ�����Һ�в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ������˵���в���ȷ����________(�����)��
A����Դ�е�aһ��Ϊ������bһ��Ϊ����
B��������NaCl��Һ��Ϊ�������Һ
C��A��B���˶������������缫
D�����������ķ�Ӧ��2H����2e��===H2��

(1)2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(g) ��H����1453.28 kJ/mol
(2)����
(3)C
(2)����
(3)C

��ϰ��ϵ�д�
�����Ŀ

 CH3OH(g) ��H=
CH3OH(g) ��H=

 �״�(CH3OH)��һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϡ���֪CH3OH(l)O2(g)=CO(g)+2H2O(g) ��Ha=-443.64KJ?mol-1 2CO(g)+O2(g)=2CO2(g)
�״�(CH3OH)��һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϡ���֪CH3OH(l)O2(g)=CO(g)+2H2O(g) ��Ha=-443.64KJ?mol-1 2CO(g)+O2(g)=2CO2(g)