��Ŀ����
��1 mol I2(g)��2 mol H2����ij2 L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g)![]() 2HI(g)����H>0������ƽ�⡣
2HI(g)����H>0������ƽ�⡣
HI���������w(HI)��ʱ��仯��ͼ����b��ʾ��
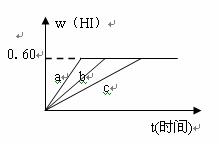
(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ___________��
(2)���ı䷴Ӧ������
�ڼ������£�w(HI)�ı仯��ͼ����a��ʾ��
���������£�w(HI)�ı仯��ͼ����c��ʾ��
�������������__________��������������___________��(�����������)
�ٺ��������£������¶� �ں��������£������¶�
�ۺ��������£���С��Ӧ������� �ܺ��������£�����Ӧ�������
�ݺ��º��������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��2 L�ܱ������м���a mol I2(g)��b mol H2��c mol HI(a��b��c������0)��������Ӧ����ƽ��ʱ��HI�����������Ϊ0.60����a��b��c�Ĺ�ϵ��________
(1)0.05 mol?L��1
(2)�ۢݢ�
(3)4a��c=2b

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ


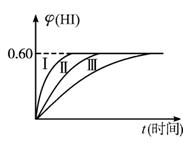
 2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ
2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ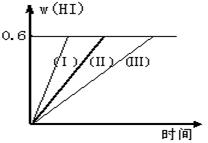
 2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��