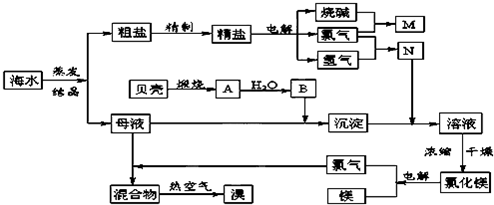
��Ŀ����
��ˮ�к��зḻ�Ļ�ѧԪ�أ�������ij�������Ӻ�ˮ����ȡNaCl��Mg����MgCl2��ʽ���ڣ���Br2����NaBr����ʽ���ڣ����ۺ����õ��������̼�ͼ��

��ش�
��1��Mg(OH)2�м��������У�Ҫ���MgCl2?6H2O���壬��Ҫ���е�ʵ���������Ϊ
��
��2��Ŀǰ��ҵ����Ҫ�������ӽ���Ĥ����ⱥ��ʳ��ˮ�����й������ӽ���Ĥ���۵������������ ��
A�����Ʊ���ʳ��ˮ���������� B����ˮ��������NaOH������������
C����������Ϊ�������ƺ����� D�����۵������ý��������Ƴ�
��3��ĸҺ�г�����MgCl2��NaCl��MgSO4��KCl�ȣ��ɽ�һ���ӹ��Ƶ�һЩ��Ҫ�IJ�Ʒ������ĸҺ�������µ�60�����ϣ�����ˮ�ֵ������������������壬�˾������Ҫ�ɷ��� �����������������Һ���µ�30�����£������������壬��һ��������ˮϴ�Ӹþ��壬���ɵõ��Ƚϴ����� ���塣
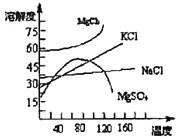
��1����������ȴ�ᾧ������
��2��D
��3��MgSO4��NaCl��KCl

��ϰ��ϵ�д�
�����Ŀ
 ��ˮ�к��зḻ�Ļ�ѧԪ�أ���ͼ��ij���������ú�ˮ������þ�����̼�ͼ����ش�
��ˮ�к��зḻ�Ļ�ѧԪ�أ���ͼ��ij���������ú�ˮ������þ�����̼�ͼ����ش�