��Ŀ����
Ԫ�����ڱ���ʾ������Ԫ�ص������Ժ͵ݱ���ɡ�ͬһ���ڹ��ɵ�ijЩ������������ͬ�ĵ������������ڱ�������Ԫ�ؿ��Թ������������Ϊ10��18�������������ڱ����г�һЩ�����ӦԪ���йص�18��������
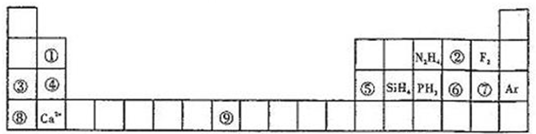
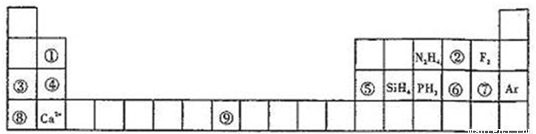
��1����Ϊ8������������Ϊ_________���ڵĵ���ʽ____________��
��2���ܢݢޢ߶�ӦԪ���γɵļ����Ӱ뾶��С�����˳��Ϊ__________________�������ӷ��ű�ʾ����
��3��д���ݶ�ӦԪ����ˮ��Ӧ�����ӷ���ʽ___________________��
��4���ܶ�Ӧ��ij��18���ӵ�������![]() ��
��![]() ���ɷ�����Ӧ��д������
���ɷ�����Ӧ��д������![]() ��Ӧ
��Ӧ
�����ӷ���ʽ:_____________________________
��5���ڻ���ƽ�����װ��Һ̬N2H4��Һ̬�ڣ���֪0.4molҺ̬N2H4������Һ̬�ڷ�Ӧ������һ����̬���ʺ�һ�ֳ���Һ̬�⻯��ų�256.6kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ________________________________________��
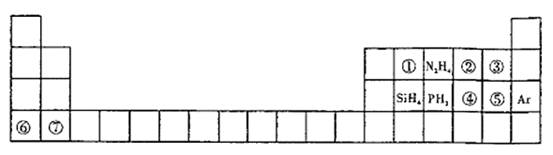
(9��) (1)���飻![]() ��
��
��2��Ca2+��K+��Cl����S2�� ��3��Cl 2+H2O=HClO + Cl��+H+
��4��HS��+OH��=S2��+H2O
��5��N2H4��l��+2H2O2��l��=N2��g��+4H2O��l�� ��H=-641.5kJ/mol
����


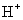 ��
��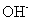 ���ɷ�����Ӧ��д������
���ɷ�����Ӧ��д������