��Ŀ����
��9�֣�������þ������ҩ��ҵ��������Ҫ����ɫ��ȼ����
I������θ�����ҩ��Stmoache����Ч�ɷ�ΪMg(OH)2��
��1����ҩ������θ�ᣨ��Ҫ�ɷ�Ϊ���ᣩ����֢ʱ��Ӧ�����ӷ���ʽ��____________________________________________��
����֪��Mg (s)+2H2O(g)=Mg(OH)2(s)��H2��g�� ��H1=��441kJ��mol��1
H2O(g)=H2(g)��![]() O2(g) ��H2=+242kJ��mol��1
O2(g) ��H2=+242kJ��mol��1
Mg(s)��![]() O2(g)=MgO(s) ��H3=��602kJ��mol��1
O2(g)=MgO(s) ��H3=��602kJ��mol��1
��2��������þ�ֽ���Ȼ�ѧ����ʽ��_______________________________________��
��3��������þ������Ϊ��ȼ����ԭ��____________________����дһ�����ɣ�
��ij��������ˮ���Ȼ�þ�ʹ�ʯ����ȡ��������þ�������������������ʣ�ͨ���������̽����ᴿ
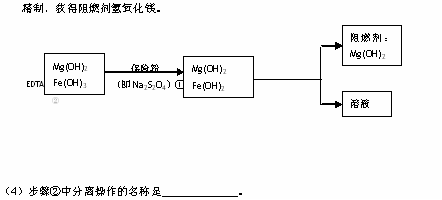
��5��������еķ�Ӧ���£�6Fe(OH)3 ��S2O42����2OH�� ��6Fe(OH)2 ��2SO42����4H2O��
ÿ����0.1mol���շۣ�Na2S2O4��ʱ��ת�Ƶ��ӵ���Ŀ�� NA��
��6����֪EDTAֻ������Һ�е�Fe2+��Ӧ����������ˮ�����ʣ�����Mg(0H)2��Ӧ����ȻFe(OH)2������ˮ���������������EDTA�ļ��룬�����ܹ���Fe(OH)2��ȥ����ô��ȸߵ�Mg(OH)2����ӳ����ܽ�ƽ��ĽǶȼ��Խ��͡�
��___________________________________________________________________��
��9�֣�
��1��Mg(OH)2+2H+=Mg2++2H2O ��1�֣�
��2��Mg(OH)2(s)=MgO(s)+H2O(g) ��H=+81kJ��mol-1 ��2�֣�
��3��������þ�ֽ�Ҫ���մ������ȣ�����������Ҳ���ո��֣�1�֣�
��4������ ��1�֣�
��5��0.6 ��2�֣�
��6��Fe(OH)2����Һ�д�������ƽ�⣺Fe(OH)2 ![]() Fe2++2OH-�������ϵ���EDTAʱ��EDTA�����Fe2+����ʹƽ�⳯���ƶ����ܽ� ��2�֣�
Fe2++2OH-�������ϵ���EDTAʱ��EDTA�����Fe2+����ʹƽ�⳯���ƶ����ܽ� ��2�֣�

(15��)������þ������ҩ��ҵ��������Ҫ����ɫ��ȼ����
������θ�����ҩ��Stmoache����Ч�ɷ�ΪMg(OH)2��
(1)��ҩ������θ��(��Ҫ�ɷ�Ϊ����)����֢ʱ��Ӧ������ʽ����ʽΪ ��
����֪:
H2O(g)=H2(g)+ O2(g) ��H1 = +242 kJ��mol-1
Mg(s)+2 H2O(g)=Mg(OH)2(s) + H2(g) ��H2 = -441 kJ��mol-1
Mg(s)+ O2(g)=MgO(s) ��H3= -602kJ��mol-1
(2)������þ�ֽ���Ȼ�ѧ����ʽΪ ��
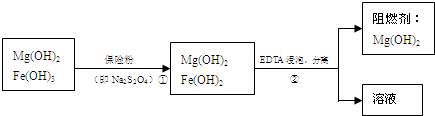
��ij��������ˮ���Ȼ�þ�ʹ�ʯ����ȡ��������þ�������������������ʣ�ͨ���������̽����ᴿ���ƣ������ȼ��������þ��
(3)������м��뱣�շ������ã� ��
(4)��֪EDTAֻ������Һ�е���Ӧ����������ˮ�����ʣ�����
��Ӧ����Ȼ
������ˮ���������������EDTA�ļ��룬�����ܹ���
��ȥ����ô��ȸߵ�
����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� ��
����Ϊ�о���ͬ�����ᴿ���������Ƶ���ȼ���Ĵ��ȴӶ�ȷ������ᴿ������ij�о�С���ȡ������������4���������Ƶõ���ȼ�����к������IJⶨ��������£�
| ������ȼ�������� | ��ȼ�������� | |||
| ��� | �ᴿ��ϵ�¶�/�� | ����EDTA����/g | ���뱣�շ�����/g | W(Fe)/(10-4g) |
| 1 | 40 | 0.05 | 0.05 | 7.63 |
| 2 | 40 | 0.05 | 0.10 | 6.83 |
| 3 | 60 | 0.05 | 0.10 | 6.83 |
| 4 | 60 | 0.10 | 0.10 | 6.51 |
(5)�����������������������ϱ����ݣ���ȡ�ߴ�����ȼ����������� (����ĸ)��
��40�� ��60�� ��EDTA����ΪO.05g
��EDTA����Ϊ0.10g �ݱ��շ�����Ϊ0.05g �ޱ��շ�����Ϊ0.10g
A���٢ۢ� B���ڢܢ� C���٢ܢ� D���ڢۢ�
(15��)������þ������ҩ��ҵ��������Ҫ����ɫ��ȼ����
������θ�����ҩ��Stmoache����Ч�ɷ�ΪMg(OH)2��
(1)��ҩ������θ��(��Ҫ�ɷ�Ϊ����)����֢ʱ��Ӧ������ʽ����ʽΪ ��
����֪:
H2O(g)=H2(g)+  O2(g)
��H1 = +242 kJ��mol-1
O2(g)
��H1 = +242 kJ��mol-1
Mg(s)+2 H2O(g)=Mg(OH)2(s) + H2(g) ��H2 = -441 kJ��mol-1
Mg(s)+  O2(g)=MgO(s)
��H3
= -602kJ��mol-1
O2(g)=MgO(s)
��H3
= -602kJ��mol-1
(2)������þ�ֽ���Ȼ�ѧ����ʽΪ ��
��ij��������ˮ���Ȼ�þ�ʹ�ʯ����ȡ��������þ�������������������ʣ�ͨ���������̽����ᴿ���ƣ������ȼ��������þ��

(3)������м��뱣�շ� �����ã�
��
�����ã�
��
(4)��֪EDTAֻ������Һ�е� ��Ӧ����������ˮ�����ʣ�����
��Ӧ����������ˮ�����ʣ����� ��Ӧ����Ȼ
��Ӧ����Ȼ ������ˮ���������������EDTA�ļ��룬�����ܹ���
������ˮ���������������EDTA�ļ��룬�����ܹ��� ��ȥ����ô��ȸߵ�
��ȥ����ô��ȸߵ� ����ӳ����ܽ�ƽ��ĽǶȼ��Խ���
��
����ӳ����ܽ�ƽ��ĽǶȼ��Խ���
��
����Ϊ�о���ͬ�����ᴿ���������Ƶ���ȼ���Ĵ��ȴӶ�ȷ������ᴿ������ij�о�С���ȡ������������4���������Ƶõ���ȼ�����к������IJⶨ��������£�
|
������ȼ�������� |
��ȼ�������� |
|||
|
��� |
�ᴿ��ϵ�¶�/�� |
����EDTA����/g |
���뱣�շ�����/g |
W(Fe)/(10-4g) |
|
1 |
40 |
0.05 |
0.05 |
7.63 |
|
2 |
40 |
0.05 |
0.10 |
6.83 |
|
3 |
60 |
0.05 |
0.10 |
6.83 |
|
4 |
60 |
0.10 |
0.10 |
6.51 |
(5)�����������������������ϱ����ݣ���ȡ�ߴ�����ȼ����������� (����ĸ)��
��40�� ��60�� ��EDTA����ΪO.05g
��EDTA����Ϊ0.10g �ݱ��շ�����Ϊ0.05g �ޱ��շ�����Ϊ0.10g
A���٢ۢ� B���ڢܢ� C���٢ܢ� D���ڢۢ�

 O2(g) ��H1 =" +242" kJ��mol-1
O2(g) ��H1 =" +242" kJ��mol-1 O2(g)="MgO(s) " ��H3 = -602kJ��mol-1
O2(g)="MgO(s) " ��H3 = -602kJ��mol-1
 �����ã� ��
�����ã� �� ��Ӧ����������ˮ�����ʣ�����
��Ӧ����������ˮ�����ʣ����� ��Ӧ����Ȼ
��Ӧ����Ȼ ������ˮ���������������EDTA�ļ��룬�����ܹ���
������ˮ���������������EDTA�ļ��룬�����ܹ��� ��ȥ����ô��ȸߵ�
��ȥ����ô��ȸߵ� ����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� ��
����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� ��