��Ŀ����
��14�֣���ij��A����Է�������Ϊ84���ش��������⣺
��1������������A�����������ϣ��������ʵ���һ�������ȼ������������������ȵ���(�����)____ ____��
a��C7H12O2 b��C6H14 c��C6H14O d��C7H14O3
��2������AΪ��������HBr�ӳɺ�ֻ�ܵõ�һ�ֲ���Ҹ�����һ�ȴ���ֻ��һ�֡�
��A�Ľṹ��ʽΪ_______ _________��
������A��Br2��CCl4��Һ��Ӧ����B��B��NaOH�Ĵ���Һ���ȿɵõ�D��D����������ԭ�ӡ���д����B�Ʊ�D�Ļ�ѧ����ʽ��______ ____________��
��B������NaOHˮ��Һ��ȫ��Ӧ�������л���E���÷�Ӧ�Ļ�ѧ����ʽ��____________ ___________________��E���Ҷ����Ĺ�ϵ��______ _________��
��3�����˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ3��2��1����A������Ϊ__________ ________��
��4����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���A�Ľṹ��ʽΪ
��ÿ��2�֣���14�֣�
��1��b��
��2��
�� (CH3)2C=C(CH3)2��
��(CH3)2CBr-CBr (CH3)2+2NaOH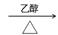 CH2=C(CH3)-C(CH3)= CH2+2NaBr+2H2O��
CH2=C(CH3)-C(CH3)= CH2+2NaBr+2H2O��
��(CH3)2CBr-CBr (CH3)2+2NaOH (CH3)2C(OH)-C(OH) (CH3)2+2NaBr��ͬϵ�
(CH3)2C(OH)-C(OH) (CH3)2+2NaBr��ͬϵ�
��3��3-��ϩ��2-�һ�-1-��ϩ�����һ�ּ��ɣ���4��
��������
���������A����Է�������Ϊ84����84/12=7����д��ѧʽֻ����C6H12��5��C���µľݱ�������CH��ɱ��ų�������Ϊϩ����
��1����ABCD�еĻ�ѧʽ����ȥO��������һ��̼ȥ����O������Hȥһ��O�������²����� C6H12��ȣ�����ACD����д��C6H12��ʽ����ѡB
��2����Ϊ����̼ԭ����ͬһƽ���ϣ����뵽��ϩ�Ľṹ������ϩ�ϵ�Hȫ������C���� (CH3)2C=C(CH3)2
(CH3)2CBr-CBr (CH3)2+2NaOH CH2=C(CH3)-C(CH3)= CH2+2NaBr+2H2O
CH2=C(CH3)-C(CH3)= CH2+2NaBr+2H2O
(CH3)2CBr-CBr
(CH3)2+2NaOH (CH3)2C(OH)-C(OH) (CH3)2+2NaBr ͬϵ��
(CH3)2C(OH)-C(OH) (CH3)2+2NaBr ͬϵ��
��3���������ֵ��˵��H�Ļ��������֣�˫�����м� CH3CH2CH=CHCH2CH3 3-��ϩ
(4) A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���A�Ľṹ��ʽֻ��Ϊ�����顣
���㣺�л�����ʽ��ȷ�����л���������ƶϡ�
��������д����������������������ʡ�







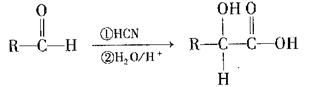
 ������R������
������R������
 ����д�������������������ʵ�һ�ֽṹ��ʽ
����д�������������������ʵ�һ�ֽṹ��ʽ
 ����ij�ϳ�������
����ij�ϳ�������  ����ԭ��֮һ����صĺϳ�·������ͼ��ʾ��ijЩ����������ȥ����
����ԭ��֮һ����صĺϳ�·������ͼ��ʾ��ijЩ����������ȥ����
 ��R1��R2��R3����������
��R1��R2��R3����������



 ��X�Ľṹ��ʽΪ
��X�Ľṹ��ʽΪ



 ������R������
������R������



 ����д�������������������ʵ�һ�ֽṹ��ʽ
��
����д�������������������ʵ�һ�ֽṹ��ʽ
�� ���ױ�����Q�����������ֻ�ѧ������ͬ����ԭ�ӣ����������ʾ�������ԭ�Ӹ�����Ϊ1��3����ش��������⣺
���ױ�����Q�����������ֻ�ѧ������ͬ����ԭ�ӣ����������ʾ�������ԭ�Ӹ�����Ϊ1��3����ش��������⣺


 ����д�������������������ʵ�һ�ֽṹ��ʽ ��
����д�������������������ʵ�һ�ֽṹ��ʽ ��