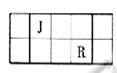
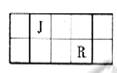
��Ŀ����
��֪��J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�L�������̬�⻯���ˮ��Һ�Լ��ԣ�M�ǵؿ��к������Ľ���Ԫ�أ�R�γɼ�������R2-��Ar�ĵ��Ӳ�ṹ��ͬ������1��M�����ӵ���ʽΪ______��
��2��Ԫ��T�����ڱ��е�λ����______��
��3���õ���ʽ��ʽJR2���γɹ���______��
��4��M��T�γɵĻ����MT3���ڳ�ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ��______������ʾ���˷�ӦΪ��������ԭ��Ӧ����
��5�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2 �����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ______��
���𰸡�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�˵��J����ͻ��ϼ�������ϼ۾���ֵ��ȣ���J����������Ϊ4��L�������̬�⻯���ˮ��Һ�Լ��ԣ���LΪNԪ�أ���ԭ��������ϵ��֪JΪCԪ�أ�R�γɼ�������R2-��Ar�ĵ��Ӳ�ṹ��ͬ����RӦΪSԪ�أ�M�ǵؿ��к������Ľ���Ԫ�أ�ӦΪAlԪ�أ���T��ԭ����������Ԫ�أ���Ϊ����������Ԫ�أ�ӦΪClԪ�أ�����Ԫ�������ɵĵݱ���ɽ����⣮
����⣺JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�˵��J����ͻ��ϼ�������ϼ۾���ֵ��ȣ���J����������Ϊ4��L�������̬�⻯���ˮ��Һ�Լ��ԣ���LΪNԪ�أ���ԭ��������ϵ��֪JΪCԪ�أ�R�γɼ�������R2-��Ar�ĵ��Ӳ�ṹ��ͬ����RӦΪSԪ�أ�M�ǵؿ��к������Ľ���Ԫ�أ�ӦΪAlԪ�أ���T��ԭ����������Ԫ�أ���Ϊ����������Ԫ�أ�ӦΪClԪ�أ�
��1��MΪAl����Ӧ���ӵĵ���ʽΪAl3+���ʴ�Ϊ��Al3+��
��2��TΪClԪ�أ�ԭ������Ϊ17��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ7��λ�����ڱ��������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��3��JR2ΪCS2���õ���ʽ�ɱ�ʾ�γɹ���Ϊ ��
��
�ʴ�Ϊ��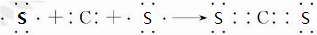 ��
��
��4��AlCl3�ɷ���ˮ�⣬����Al��OH��3��HCl��HCl��Ͽ�����ˮ�������γɰ���������ʽΪAlCl3+3H2O?Al��OH��3+3HCl��
�ʴ�Ϊ��AlCl3+3H2O?Al��OH��3+3HCl��
��5����Ϊ��ˮ��Һ����H2O2��Ӧ���ɵIJ��ﲻ��Ⱦ��������Ӧ����N2��H2O����Ӧ�Ļ�ѧ����ʽΪ2NH3?H2O+3H2O2�TN2+8H2O��
�ʴ�Ϊ��2NH3?H2O+3H2O2�TN2+8H2O��
���������⿼��Ԫ��λ�ýṹ�����ʣ���Ŀ�Ѷ��еȣ�����ע�����ԭ�ӽṹ��������Ԫ�ض�Ӧ���ʡ����������;�����ʴ�Ϊ�ƶϸ���Ĺؼ�����Ҫ������ѧϰ��ע����ۣ�
����⣺JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�˵��J����ͻ��ϼ�������ϼ۾���ֵ��ȣ���J����������Ϊ4��L�������̬�⻯���ˮ��Һ�Լ��ԣ���LΪNԪ�أ���ԭ��������ϵ��֪JΪCԪ�أ�R�γɼ�������R2-��Ar�ĵ��Ӳ�ṹ��ͬ����RӦΪSԪ�أ�M�ǵؿ��к������Ľ���Ԫ�أ�ӦΪAlԪ�أ���T��ԭ����������Ԫ�أ���Ϊ����������Ԫ�أ�ӦΪClԪ�أ�
��1��MΪAl����Ӧ���ӵĵ���ʽΪAl3+���ʴ�Ϊ��Al3+��
��2��TΪClԪ�أ�ԭ������Ϊ17��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ7��λ�����ڱ��������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��3��JR2ΪCS2���õ���ʽ�ɱ�ʾ�γɹ���Ϊ
 ��
���ʴ�Ϊ��
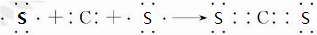 ��
����4��AlCl3�ɷ���ˮ�⣬����Al��OH��3��HCl��HCl��Ͽ�����ˮ�������γɰ���������ʽΪAlCl3+3H2O?Al��OH��3+3HCl��
�ʴ�Ϊ��AlCl3+3H2O?Al��OH��3+3HCl��
��5����Ϊ��ˮ��Һ����H2O2��Ӧ���ɵIJ��ﲻ��Ⱦ��������Ӧ����N2��H2O����Ӧ�Ļ�ѧ����ʽΪ2NH3?H2O+3H2O2�TN2+8H2O��
�ʴ�Ϊ��2NH3?H2O+3H2O2�TN2+8H2O��
���������⿼��Ԫ��λ�ýṹ�����ʣ���Ŀ�Ѷ��еȣ�����ע�����ԭ�ӽṹ��������Ԫ�ض�Ӧ���ʡ����������;�����ʴ�Ϊ�ƶϸ���Ĺؼ�����Ҫ������ѧϰ��ע����ۣ�

��ϰ��ϵ�д�
�����Ŀ