��Ŀ����
��ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
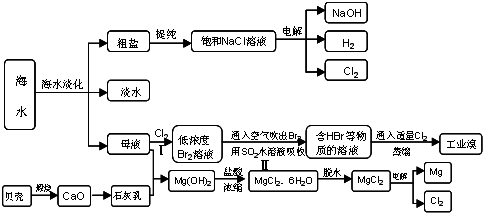
�� ��ˮ�к��д������Ȼ��ơ���д�������ӽṹʾ��ͼ ��
�� Ŀǰ������ʹ�õġ���ˮ��������Ҫ����֮һ�����������ǽ���ˮ�������������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж������� ���������仯����ѧ�仯����
�� ��ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ������ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ ��
�� ���������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷������������£�

��֪SO2��CO2�����������������д���ڢܵĻ�ѧ��Ӧ����ʽ��
��__________________________________����_____________________________________��
���� ��1�֣�
�������仯 ��1�֣�
��2NaCl+2H2O = 2NaOH��H2����Cl2�� ��2�֣�
��SO2��NaOH��NaHSO3 ��2�֣� NaHSO3��HCl��NaCl��SO2����H2O ��2�֣�
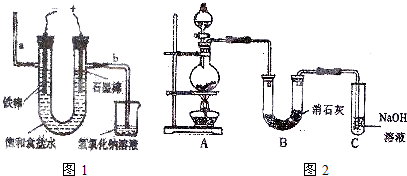
����������

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�