��Ŀ����
ijʵ����Ҫ100 mL��1 mol/L��Na2CO3��Һ����ͨ�����²������ƣ�
�� �ѳ����õĹ���Na2CO3����С�ձ��У�����������ˮ�ܽ⡣Ϊ�ӿ��ܽ����ʹ�� �����������ƣ����� �ڰѢ�������Һ��ȴ�����º�С��ת�� �����������ƣ��ۼ���������ˮ��Һ�����̶���1��2cm�������� �����������ƣ�С�ĵμ�����ˮ����Һ��Һ����͵���̶������Т�����������ˮϴ�Ӳ��������ձ�2��3�Σ�ÿ��ϴ�ӵ���Һ��С��ת������ƿ��������ҡ�Ȣݽ�����ƿ���������ҡ�ȡ�
��1������������ȷ��˳���� ������ţ���
��2����û�в����ܣ���������Һ��Ũ�Ȼ� ���ƫ�ߡ���ƫ�͡�����
��3����������Һ���ܶ�Ϊ1.06 g/mL�������Һ����������Ϊ ��
��4����ȡ��20 mL���Na2CO3����Һ��������ˮϡ�ͳ�c(Na+)=0.01 mol/L����Һ����ϡ�ͺ���Һ�����Ϊ mL
��5��������100 mL��1 mol/L��Na2CO3��Һʱ�����в����е� �ᵼ�½��ƫ�ͣ����������д��
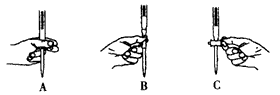
����������ƽ����ʱ�����������
�ڽ��ձ��е���Һת�Ƶ�����ƿʱ������������ƿ��
�۶���ʱ���ӿ̶���
�ܶ���ʱ���ӿ̶���
�ݸɾ�������ƿδ�����������������Һ
�� ������ ��1�֣�
�� 100 mL����ƿ ��1��,û��д���,������;����д��,������.��
�� ��ͷ�ι� ��1�֣�
��1�� ���������� ��1�֣�
��2�� ƫ�� ��1�֣���
��3�� 10% ��1�֣���
��4�� 4000 ��1�֣�
��5�� ������ ��2��,©ѡ������.��
����:��