��Ŀ����
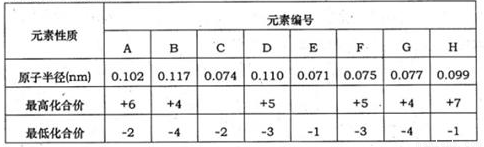
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ���Ӧ�⻯��е�����ݣ�
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| ԭ�Ӱ뾶 | 0.102 | 0.075 | 0.117 | 0.074 | 0.110 | 0.071 | 0.099 | 0.077 |
| ����ϼ� | +6 | +5 | ��4 | +5 | +7 | ��4 | ||
| ��ͻ��ϼ� | ��2 | ��3 | ��4 | ��2 | ��3 | ��1 | ��1 | ��4 |
��֪����A��D���γɻ�����AD2��AD3����B��D���γɶ��ֻ�����,����BD��BD2�dz����Ļ�����,C�������ƹ��ء�
��ش𣺡�
��1��E�����ڱ���λ���� ��
��2��C��H����̬�⻯����ȶ���ǿ����ϵΪ: (�÷���ʽ��ʾ)
��3��32g AD2�����D2����ǡ����ȫ��Ӧ����AD3���壬�ų�49.15kJ�����������䷴Ӧ�Ȼ�ѧ����ʽΪ��
��4���������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᡣ�÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��5����ҵ�Ͽ��ô�����Һ����BD��BD2���÷�Ӧ���£�
BD+BD2+Na2CO3=2 ������+CO2������ij�εĻ�ѧʽӦΪ������������
(1).��������,VA��
(2).CH4>SiH4
(3).2SO2(g) + O2(g) = 2SO3(g) ��H��-196.6 kJ/mol
(4).SOCl2 + H2O = SO2 + 2HCl
(5)NaNO2(ÿС��3��)

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�

 �±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺