��Ŀ����
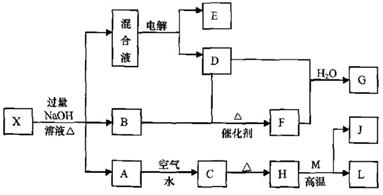
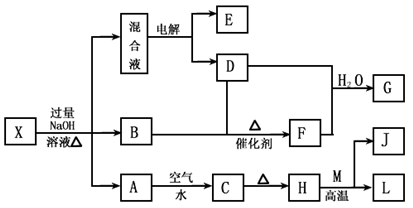
��һ������X����ˮ��ҺΪdz��ɫ���ɷ������µ�ת����ϵ�����ַ�Ӧ����������ԣ������У�B��D��E��F��Ϊ��ɫ���壬M��LΪ�����Ľ������ʣ�CΪ������ˮ�ĺ��ɫ���塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��M��Ӧ�ɷų��������ȡ�
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2������Ԫ��M��ԭ�ӽṹʾ��ͼ ��
��3������X�Ļ�ѧʽΪ ��
��4�������Һʱ������ӦʽΪ ��
��5����Ҫ��д������ת����ϵ���йط�Ӧ�Ļ�ѧ����ʽ��
�� ����LԪ�صĻ��Ϸ�Ӧ ��
�� ����LԪ�ص��û���Ӧ ��
��6����֪![]()
 E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�![]() kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
��1�� ��2�֣� ��2��
��2�֣�
��3����NH4��2Fe��SO4��2��2�֣�
��4��4OH
![]() 4e
4e![]() ====O2��+2H2O ��2�֣�
====O2��+2H2O ��2�֣�
��5��4Fe��OH��2+O2+2H2O=====4Fe��OH��3��2�֣���
Fe2O3+2Al2Fe+A12O3 ��2�֣�
��6��H2��g��+![]() O2��g��=====H2O��1������H=
O2��g��=====H2O��1������H=![]() kJ/mol ��2�֣�
kJ/mol ��2�֣�
����:��

 ABC����ȫ�ž�ϵ�д�
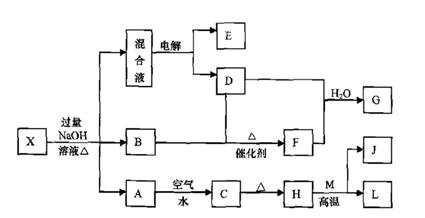
ABC����ȫ�ž�ϵ�д���һ������X����ˮ��ҺΪdz��ɫ���ɷ������µ�ת����ϵ�����ַ�Ӧ����������ԣ������У�B��D��E��F��Ϊ��ɫ���壬M��LΪ�����Ľ������ʣ�CΪ������ˮ�ĺ��ɫ���塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��M��Ӧ�ɷų��������ȡ�
��ش��������⣺
 |
��1��B�ĵ���ʽΪ ��
��2������Ԫ��M��ԭ�ӽṹʾ��ͼ ��
��3������X�Ļ�ѧʽΪ ��
��4�������Һʱ������ӦʽΪ ��
��5����Ҫ��д������ת����ϵ���йط�Ӧ�Ļ�ѧ����ʽ��
�� ����LԪ�صĻ��Ϸ�Ӧ ��
�� ����LԪ�ص��û���Ӧ ��
��6����֪![]()
![]() E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�![]() kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
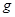 E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�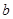 kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ
��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ
��
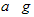 E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�b kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ________________________ ��
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�b kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ________________________ ��