��Ŀ����
��֪�� 

�л���A ~F�У�A��������A��H2�ӳɵõ����������ⶨ��������������ֻ������-CH3-����һ±����������ͬ���칹�壬C��X��Ӧ����D��������֮�ǵ�ת����ϵ����

��1��д��X�����ƣ�___________��
��2��д��������A��E�Ľṹ��ʽ��A_________��E___________��
��3��ָ�����з�Ӧ�����ͣ�C��D___________________��E��F___________________��
��4��д��B����������������ͭ����Һ����ʱ������Ӧ�Ļ�ѧ����ʽ��______________________��
��5���л���G���л���C��ͬ���͵�ͬ���칹�壬����G��������Ʒ�����Ӧ�ų���ɫ���壬���ܷ���������Ӧ����֪G��ͬһ���͵�ͬ���칹�干�ж��֣�������ֻ����һ������ͬ���칹��Ľṹ��ʽ��
___________��____________������д���֣�
��2��д��������A��E�Ľṹ��ʽ��A_________��E___________��
��3��ָ�����з�Ӧ�����ͣ�C��D___________________��E��F___________________��
��4��д��B����������������ͭ����Һ����ʱ������Ӧ�Ļ�ѧ����ʽ��______________________��
��5���л���G���л���C��ͬ���͵�ͬ���칹�壬����G��������Ʒ�����Ӧ�ų���ɫ���壬���ܷ���������Ӧ����֪G��ͬһ���͵�ͬ���칹�干�ж��֣�������ֻ����һ������ͬ���칹��Ľṹ��ʽ��
___________��____________������д���֣�
��1���Ҵ�
��2��A��CH3CH2CH=CH2��E��CH3CH=C(CH3)COOCH2CH3
��3��������Ӧ��ȡ�������Ӿ۷�Ӧ
��2��A��CH3CH2CH=CH2��E��CH3CH=C(CH3)COOCH2CH3
��3��������Ӧ��ȡ�������Ӿ۷�Ӧ
��4��CH3CH=C(CH3)CHO+2Cu(OH)2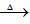 CH3CH=C(CH3)COOH+2H2O+Cu2O��
CH3CH=C(CH3)COOH+2H2O+Cu2O��
��5��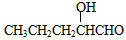 ��
�� ��
��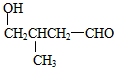 ���������ּ��ɣ�
���������ּ��ɣ�
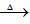 CH3CH=C(CH3)COOH+2H2O+Cu2O��
CH3CH=C(CH3)COOH+2H2O+Cu2O�� ��5��
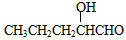 ��
�� ��
��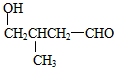 ���������ּ��ɣ�
���������ּ��ɣ�
��ϰ��ϵ�д�
�����Ŀ
��֪ij�л���A�ĺ�����ͺ˴Ź���������ͼ������˵������ȷ���ǣ�������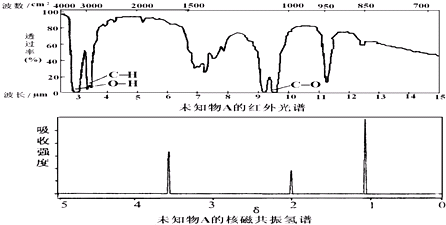

| A���ɺ������֪�����л��������������ֲ�ͬ�Ļ�ѧ�� | B��������˴Ź�����������֪������е���ԭ������ | C����A�Ļ�ѧʽΪC3H6O������ṹ��ʽΪCH3COCH3 | D���ɺ˴Ź�������֪�����л�������������ֲ�ͬ��ѧ��������ԭ�� |
��֪ij�л���A�ķ���ʽ��C2H4���ܷ�������ת����ϵ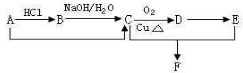 �����ַ�Ӧ��������P��Ӧ��������ȥ��������˵����ȷ���ǣ�������
�����ַ�Ӧ��������P��Ӧ��������ȥ��������˵����ȷ���ǣ�������
 �����ַ�Ӧ��������P��Ӧ��������ȥ��������˵����ȷ���ǣ�������
�����ַ�Ӧ��������P��Ӧ��������ȥ��������˵����ȷ���ǣ�������| A����ת����ϵ����������Ӧ��ȡ����Ӧ | B��B��Ũ������ȵ�������������A | C��A��C��D����ʹ���Ը��������Һ��ɫ | D����F��F��ͬ���칹��������F��ͬϵ��ֻ��1�� |



